Figure 2.
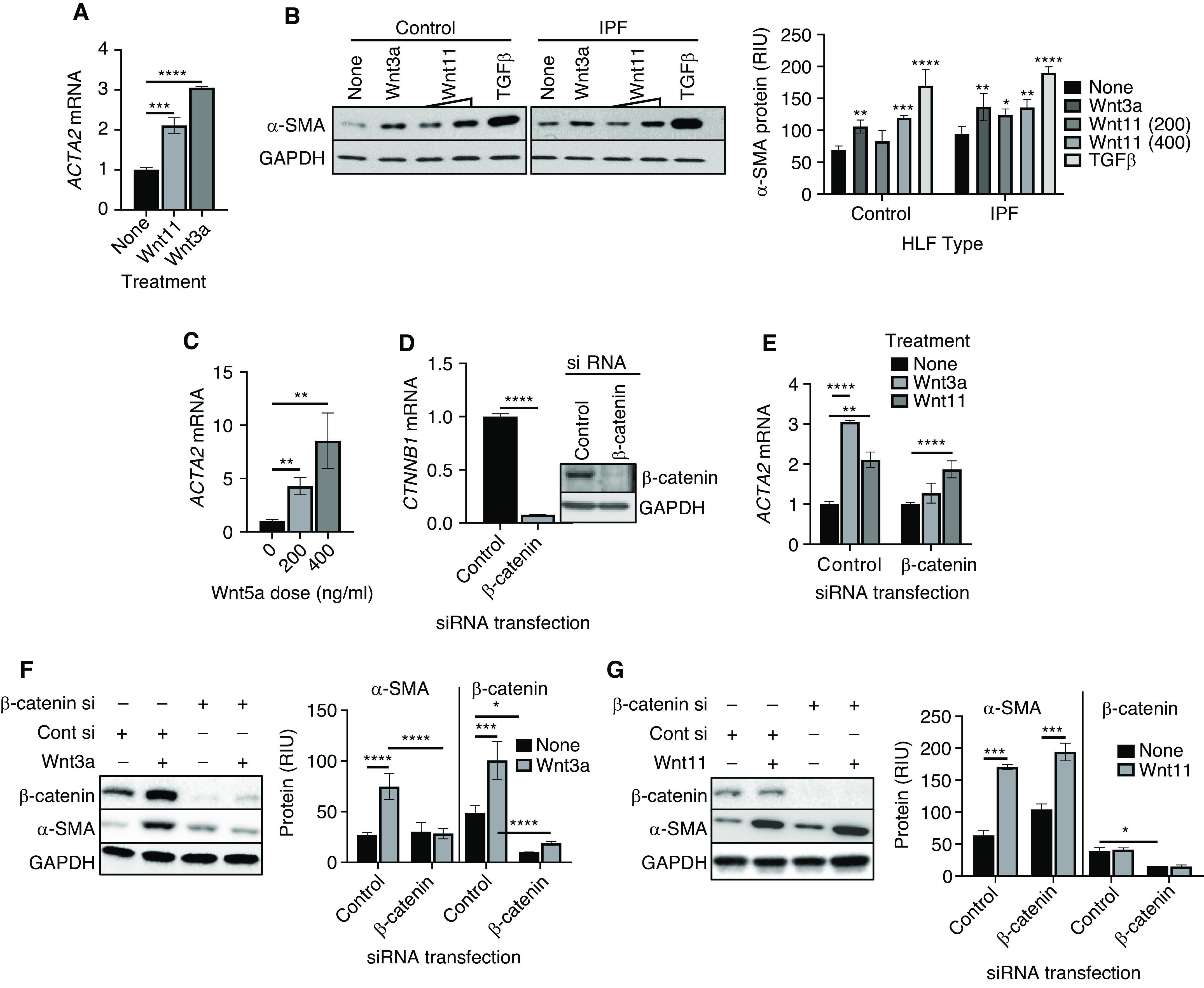
Wnt11 induction of myofibroblast differentiation is β-catenin independent. (A) Normal human lung fibroblasts (HLFs) treated with 200 ng/ml Wnt 3a or 400 ng/ml Wnt11 were analyzed for α-SMA (ACTA2) mRNA by using qPCR analysis, and the results were expressed as the fold change over that in untreated cells. (B) The protein level of α-SMA in HLFs isolated from Cont subjects or patients with IPF treated with Wnt3a (200 ng/ml), Wnt11 (200 and 400 ng/ml), or TGFβ (5 ng/ml) was assayed by using Western blotting. The GAPDH signal was used as internal Cont. The quantified protein expression level was expressed in RIU after normalization to GAPDH signals (bottom). Representative blots from at least three separate experiments are shown. (C) The mRNA level of ACTA2 was analyzed in HLFs treated with Wnt5a after 24 hours. (D) HLFs were transfected with Cont siRNA (Cont si) or siRNA against β-catenin (β-catenin si) as indicated, and the β-catenin mRNA (CTNNB1) and protein in the HLFs were analyzed by using qPCR (left panel) and Western blotting (right panel) analysis. (E) HLFs were treated with Wnt3a or Wnt11 after 16 hours of transfection with Cont si or β-catenin si for another 24 hours and were then analyzed for the ACTA2 gene. With the same treatment used in E, the proteins were analyzed by using Western blotting for HLF β-catenin and α-SMA protein after (F) Wnt3a treatment or (G) β-catenin si and/or Wnt11 treatment, with respective bar graphs summarizing the quantitative analyses. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 between the two indicated groups (N = 3 for all groups). RIU = relative integration units.
