Abstract
Rationale & Objective
Acute kidney injury treated with kidney replacement therapy (AKI-KRT) occurs frequently in critically ill patients with coronavirus disease 2019 (COVID-19). We examined the clinical factors that determine kidney recovery in this population.
Study Design
Multicenter cohort study.
Setting & Participants
4,221 adults not receiving KRT who were admitted to intensive care units at 68 US hospitals with COVID-19 from March 1 to June 22, 2020 (the “ICU cohort”). Among these, 876 developed AKI-KRT after admission to the ICU (the “AKI-KRT subcohort”).
Exposure
The ICU cohort was analyzed using AKI severity as the exposure. For the AKI-KRT subcohort, exposures included demographics, comorbidities, initial mode of KRT, and markers of illness severity at the time of KRT initiation.
Outcome
The outcome for the ICU cohort was estimated glomerular filtration rate (eGFR) at hospital discharge. A 3-level outcome (death, kidney nonrecovery, and kidney recovery at discharge) was analyzed for the AKI-KRT subcohort.
Analytical Approach
The ICU cohort was characterized using descriptive analyses. The AKI-KRT subcohort was characterized with both descriptive analyses and multinomial logistic regression to assess factors associated with kidney nonrecovery while accounting for death.
Results
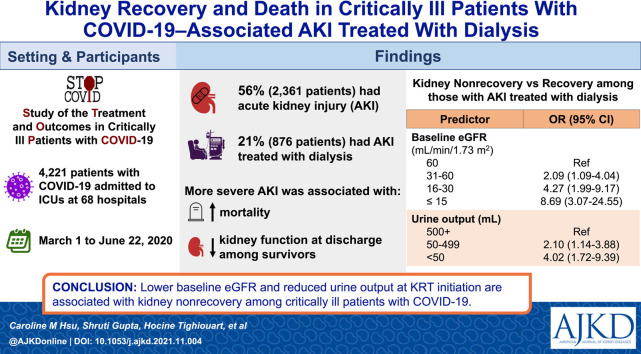
Among a total of 4,221 patients in the ICU cohort, 2,361 (56%) developed AKI, including 876 (21%) who received KRT. More severe AKI was associated with higher mortality. Among survivors, more severe AKI was associated with an increased rate of kidney nonrecovery and lower kidney function at discharge. Among the 876 patients with AKI-KRT, 588 (67%) died, 95 (11%) had kidney nonrecovery, and 193 (22%) had kidney recovery by the time of discharge. The odds of kidney nonrecovery was greater for lower baseline eGFR, with ORs of 2.09 (95% CI, 1.09-4.04), 4.27 (95% CI, 1.99-9.17), and 8.69 (95% CI, 3.07-24.55) for baseline eGFR 31-60, 16-30, ≤15 mL/min/1.73 m2, respectively, compared with eGFR > 60 mL/min/1.73 m2. Oliguria at the time of KRT initiation was also associated with nonrecovery (ORs of 2.10 [95% CI, 1.14-3.88] and 4.02 [95% CI, 1.72-9.39] for patients with 50-499 and <50 mL/d of urine, respectively, compared to ≥500 mL/d of urine).
Limitations
Later recovery events may not have been captured due to lack of postdischarge follow-up.
Conclusions
Lower baseline eGFR and reduced urine output at the time of KRT initiation are each strongly and independently associated with kidney nonrecovery among critically ill patients with COVID-19.
Index Words: Acute kidney injury (AKI), chronic kidney disease (CKD), coronavirus disease 2019 (COVID-19), critical care, dialysis, estimated glomerular filtration rate (eGFR), oligoanuria, prognostication, renal function, renal recovery, urine output
Graphical abstract
Plain-Language Summary.
Critically ill patients with COVID-19 often develop acute kidney injury (AKI). This study of 4,221 patients demonstrated that more severe AKI was associated with greater in-hospital mortality and poorer kidney function at hospital discharge. Among the 876 patients who required dialysis for AKI, almost two-thirds died. Among those who survived to discharge, about two-thirds recovered kidney function and were discharged without the need for dialysis. Lower baseline kidney function and reduced urine output were associated with nonrecovery of kidney function. Identification of such predictors is important in assessing prognosis among these critically ill patients and has implications for clinical care of individuals critically ill with COVID-19.
Acute kidney injury (AKI) occurs frequently in patients with coronavirus disease 2019 (COVID-19), affecting 17% to 46% of hospitalized patients, 14% to 20% of whom are treated with kidney replacement therapy (KRT).1, 2, 3, 4 Dialysis is resource intensive, and long-term dialysis impacts patients’ quality of life and other clinically important outcomes.5, 6, 7 Furthermore, studies have demonstrated high mortality among critically ill patients with COVID-19 whose AKI is treated with dialysis.1 , 8 Accurate prognostication of both long-term dialysis dependence and mortality therefore has key implications for clinical outcomes and quality of life, and may affect decision-making in the setting of critical illness and AKI.
At this time, no widely accepted tools exist for prognostication of kidney recovery from AKI and in particular from AKI treated by KRT (AKI-KRT). Historically, mortality as a competing outcome has been difficult to account for.9 , 10 Studies of kidney recovery have been further limited by variation in recovery patterns and lack of detailed data on clinical status at the time of AKI or of KRT initiation.9, 10, 11 In comparison, AKI associated with COVID-19, though occurring via multiple possible mechanisms,12 has less heterogeneity with respect to timing and underlying cause; thus, a pattern for recovery may be discernable.
Using detailed clinical data from a large multicenter cohort of critically ill patients with COVID-19, we first investigated the association of AKI severity with kidney function at the time of hospital discharge. Next, among patients with AKI-KRT, we examined the clinical factors that may predict kidney recovery, using baseline characteristics and measures of clinical status at the time of dialysis initiation.
Methods
Study Design and Data Collection
We used data from the Study of the Treatment and Outcomes in Critically Ill Patients With COVID-19 (STOP-COVID), a multicenter cohort study that enrolled consecutive adults (≥18 years old) with laboratory-confirmed COVID-19 admitted to participating intensive care units (ICUs) at 68 hospitals across the United States. We included patients admitted to ICUs between March 1 and June 22, 2020. This study was approved by the institutional review boards at all participating sites with a waiver of informed consent. All data except dates were deidentified.
Study personnel at each site collected data by detailed chart review using a standardized electronic case report form via a secure Research Electronic Data Capture (REDCap) database. Patient-level data included baseline information on demographics, coexisting conditions, symptoms, medications before hospital admission, and vital signs on ICU admission; daily data for the 14 days after ICU admission on physiologic and laboratory values, medications, nonmedication treatments, and organ support; and outcomes data on discharge and death, including kidney function at discharge. Additional details regarding the STOP-COVID parent study are reported elsewhere.8 , 13
AKI Severity and Outcomes in the ICU Cohort
Initial analyses evaluated the entire STOP-COVID population, excluding those receiving dialysis at hospital admission, those without outcome data, those still hospitalized at last follow-up, those without kidney function assessment at discharge, and those without a baseline serum creatinine concentration (Scr); these patients composed the ICU cohort. These descriptive analyses examined kidney outcomes and mortality by severity of AKI. Baseline Scr was defined as the lowest of 3 values: (1) lowest preadmission Scr value between 7 and 365 days before admission; (2) Scr on hospital admission; and (3) Scr on ICU day 1. AKI severity was determined by calculating the ratio of the peak Scr in the first 14 days after ICU admission relative to the baseline value, using thresholds defined by KDIGO AKI staging.14 Similarly, among survivors, the ratio of the discharge Scr to the baseline as well as ongoing treatment with dialysis at discharge was used to categorize kidney recovery. Mortality was defined by vital status at hospital discharge. Outcomes were compared by AKI severity.
Kidney Outcomes and Mortality in the AKI-KRT Subcohort
Patient Population and Exposures
Further analysis focused on the subset of patients who received dialysis during the first 14 days after ICU admission, termed the AKI-KRT subcohort. KRT day 1 was defined as a patient’s first day of dialysis.
Exposures of interest identified a priori based on clinical importance and availability in the dataset were demographics (age, sex, race, ethnicity), baseline medical status (history of diabetes mellitus, estimated glomerular filtration rate (eGFR) at baseline using the CKD-EPI equation with a race coefficient),15 initial mode of dialysis (continuous kidney replacement therapy [CKRT], intermittent hemodialysis, or peritoneal dialysis), markers of illness severity on KRT day 1 (serum albumin, arterial pH, 24-hour urine output, maximum number of vasopressors or inotropes received that day), and occurrence of a major cardiac event (ventricular tachycardia, ventricular fibrillation, or cardiac arrest) on or preceding KRT day 1.
Outcomes
Patients were followed until either hospital discharge or death. Kidney recovery was defined as independence from dialysis at discharge. The date of recovery was the date of last dialysis; if it could not be determined (because daily data on KRT were only collected for the first 14 days after ICU admission and on hospital discharge), it was set as the date of discharge (see Item S1 for details). Among survivors discharged with kidney recovery, Scr at the time of discharge was used to calculate discharge eGFR, which was then compared with the baseline eGFR in a series of descriptive analyses.
Statistical Analysis
Several regression methods were used to assess predictors of kidney recovery while accounting for hospital mortality. These regression analyses were applied to the same group of patients, the AKI-KRT subcohort, to assess for consistency in results. Missing exposure variables were imputed using methods described in Item S1.
Primary analyses used multinomial logistic regression to associate covariates of interest with the outcome of kidney recovery, using kidney nonrecovery as the reference outcome and death as an alternate outcome.
Two secondary analyses were conducted. First, ordinal logistic regression analysis was performed to associate covariates with the composite outcome of kidney nonrecovery or death and with the outcome of death alone, thus treating death as the least desirable outcome, followed by kidney nonrecovery with kidney recovery as the most desirable outcome. Second, because logistic regression does not account for differences in follow-up time, instead assuming the same follow-up time for all patients, we used cause-specific time-to-recovery analysis to assess the instantaneous rate of recovery, treating death as a competing event. (Time-to-recovery was not used for primary analysis due to uncertainty around the date of recovery, with most patients’ recovery status being reported only at hospital discharge.) Those who were discharged without kidney recovery were censored at the time of discharge.
For each of these 3 approaches, 3 sets of analyses were conducted: (1) primary analyses used all a priori specified exposure variables; (2) parsimonious analyses included only demographic characteristics and baseline medical status assessments; and (3) expanded analyses further incorporated medications received on or before KRT day 1 (corticosteroids, tocilizumab, and remdesivir, chosen for clinical relevance) and additional assessments of clinical status on KRT day 1 (use of mechanical ventilation, the coagulation component of the SOFA [Sequential Organ Failure Assessment] score, and the liver component of the SOFA score).
Statistical analyses were performed with SAS EG v7.14 (SAS Institute).
Results
AKI Severity and Outcomes in the ICU Cohort
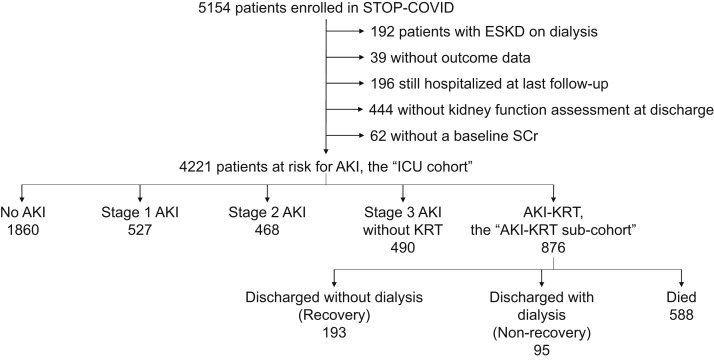
Among 5,154 patients enrolled in STOP-COVID, 741 had incomplete data reporting, and 192 were receiving maintenance dialysis at admission; therefore, 4,221 patients constituted the ICU cohort (Fig 1 ). Of these, 2,681 (63%) were male, and the mean age was 61 ± 15 (SD) years; 1,085 (26%) had a baseline eGFR of ≤60 mL/min/1.73 m2 (Table 1 ).
Figure 1.
Flow diagram of the study population. Abbreviations: AKI, acute kidney injury; ESKD, end-stage kidney disease; ICU, intensive care unit; KRT, kidney replacement therapy; Scr, serum creatinine; STOP-COVID, Study of the Treatment and Outcomes in Critically Ill Patients With COVID-19.
Table 1.
Characteristics of STOP-COVID Population at Risk for AKI, Stratified by Baseline eGFR
| Overall | Baseline eGFR, mL/min/1.73 m2 |
||||
|---|---|---|---|---|---|
| >60 | 31-60 | 16-30 | ≤15 | ||
| No. of patients | 4,221 | 3,136 (74%) | 777 (18%) | 217 (5%) | 91 (2%) |
| Age, y | 61 ± 15 | 59 ± 15 | 69 ± 12 | 68 ± 14 | 64 ± 15 |
| Male sex | 2,681 (63.5%) | 2,007 (64.0%) | 498 (64.1%) | 121 (55.8%) | 55 (60%) |
| Race | |||||
| White | 1,625 (38.5%) | 1,220 (38.9%) | 305 (39.3%) | 67 (30.9%) | 33 (36%) |
| Black or African American | 1,242 (29.4%) | 848 (27.0%) | 268 (34.5%) | 89 (41.0%) | 37 (41%) |
| Asian | 244 (5.8%) | 186 (5.9%) | 44 (5.7%) | 12 (5.5%) | 2 (2%) |
| American Indian/Alaska Native | 23 (0.5%) | 18 (0.6%) | 5 (0.6%) | 0 (0.0) | 0 (0) |
| Native Hawaiian or Other Pacific Islander | 27 (0.6%) | 17 (0.5%) | 7 (0.9%) | 2 (0.9%) | 1 (1%) |
| More than 1 race | 44 (1.0%) | 37 (1.2%) | 5 (0.6%) | 1 (0.5%) | 1 (1%) |
| Unknown/not reported | 1,016 (24.1%) | 810 (25.8%) | 143 (18.4%) | 46 (21.2%) | 17 (19%) |
| Ethnicity | |||||
| Hispanic or Latino | 1,025 (24.3%) | 850 (27.1%) | 128 (16.5%) | 33 (15.2%) | 14 (15%) |
| Not Hispanic or Latino | 2,671 (63.3%) | 1,884 (60.1%) | 567 (73.0%) | 156 (71.9%) | 64 (70%) |
| Unknown | 525 (12.4%) | 402 (12.8%) | 82 (10.6%) | 28 (12.9%) | 13 (14%) |
| Diabetes mellitus | 1,756 (41.6%) | 1,176 (37.5%) | 400 (51.5%) | 130 (59.9%) | 50 (55%) |
| Characteristics on ICU Day 1 | |||||
| Serum albumin (g/dL) | 3.20 [2.80-3.60] | 3.20 [2.80-3.60] | 3.20 [2.80-3.60] | 3.00 [2.50-3.40] | 3.00 [2.60-3.50] |
| Arterial pH | 7.37 [7.30-7.43] | 7.39 [7.31-7.44] | 7.34 [7.28-7.41] | 7.30 [7.21-7.39] | 7.27 [7.21-7.35] |
| Urine output | |||||
| ≥500 mL/d | 1,448 (34.3%) | 1,104 (35.2%) | 260 (33.5%) | 63 (29.0%) | 21 (23%) |
| 50-499 mL/d | 678 (16.1%) | 448 (14.3%) | 152 (19.6%) | 59 (27.2%) | 19 (21%) |
| <50 mL/d | 91 (2.2%) | 53 (1.7%) | 15 (1.9%) | 7 (3.2%) | 16 (18%) |
| Unknown | 2,004 (47.5%) | 1,531 (48.8%) | 350 (45.0%) | 88 (40.6%) | 35 (39%) |
| No. of vasopressors/inotropes | |||||
| 0 | 2,568 (60.8%) | 1,978 (63.1%) | 429 (55.2%) | 120 (55.3%) | 41 (45%) |
| 1 | 1,216 (28.8%) | 892 (28.4%) | 228 (29.3%) | 60 (27.6%) | 36 (40%) |
| ≥2 | 437 (10.4%) | 266 (8.5%) | 120 (15.4%) | 37 (17.1%) | 14 (15%) |
| Major cardiac event | 119 (2.8%) | 70 (2.2%) | 32 (4.1%) | 11 (5.1%) | 6 (7%) |
| Medications Administered | |||||
| Corticosteroids | 634 (15.0%) | 455 (14.5%) | 124 (16.0%) | 41 (18.9%) | 14 (15%) |
| Tocilizumab | 227 (5.4%) | 172 (5.5%) | 43 (5.5%) | 10 (4.6%) | 2 (2%) |
| Remdesivir | 130 (3.1%) | 115 (3.7%) | 13 (1.7%) | 2 (0.9%) | 0 (0) |
| Mechanical ventilation | 2,500 (59.2%) | 1,798 (57.3%) | 493 (63.4%) | 141 (65.0%) | 68 (75%) |
| Platelets | |||||
| ≥150 ×103/μL | 3,242 (76.8%) | 2,454 (78.3%) | 560 (72.1%) | 162 (74.7%) | 66 (73%) |
| 100-149 ×103/μL | 553 (13.1%) | 376 (12.0%) | 137 (17.6%) | 28 (12.9%) | 12 (13%) |
| <100 ×103/μL | 204 (4.8%) | 138 (4.4%) | 42 (5.4%) | 16 (7.4%) | 8 (9%) |
| Unknown | 222 (5.3%) | 168 (5.4%) | 38 (4.9%) | 11 (5.1%) | 5 (6%) |
| Bilirubin | |||||
| <1.2 mg/dL | 3,081 (73.0%) | 2,275 (72.5%) | 576 (74.1%) | 161 (74.2%) | 69 (76%) |
| 1.2-1.9 mg/dL | 280 (6.6%) | 214 (6.8%) | 54 (6.9%) | 8 (3.7%) | 4 (4%) |
| >2.0 mg/dL | 120 (2.8%) | 87 (2.8%) | 19 (2.4%) | 10 (4.6%) | 4 (4%) |
| Unknown | 740 (17.5%) | 560 (17.9%) | 128 (16.5%) | 38 (17.5%) | 14 (15%) |
Values for continuous variables given as mean ± SD or median [interquartile range]; for categorical data as frequency (%).
Abbreviations: AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; STOP-COVID, Study of the Treatment and Outcomes in Critically Ill Patients With COVID-19.
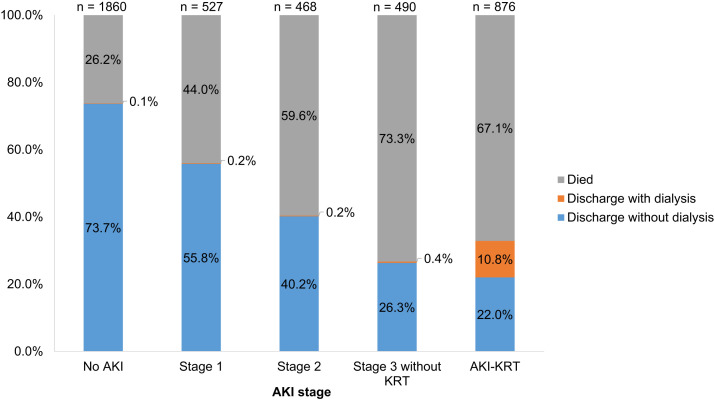
Within the first 14 days after ICU admission, 2,361 patients (56%) developed AKI, including 527 (12%) with stage 1, 468 (11%) with stage 2, 490 (12%) with stage 3 without KRT, and 876 (21%) who received KRT (Fig 1). More severe AKI was associated with greater mortality; among those with no AKI, AKI stage 1, AKI stage 2, AKI stage 3 without KRT, and AKI-KRT, 26%, 44%, 60%, 73%, and 67% died, respectively (Fig 2 ). Of those with AKI-KRT, 11% were discharged while continuing to receive dialysis. Discharge with dialysis occurred in less than 0.5% of patients with other stages of AKI; because in this study AKI stage was defined by its peak severity within the first 14 days of ICU admission, these patients must have received KRT beginning after the first 14 days of ICU admission.
Figure 2.
Outcomes of the ICU cohort. AKI stage 1 defined as peak Scr is 1.5-1.9 times baseline Scr; AKI stage 2, peak Scr is 2.0-2.9 times baseline; AKI stage 3 without KRT, peak Scr is ≥3.0 times baseline. Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; KRT, kidney replacement therapy; Scr, serum creatinine.
Among survivors, more severe AKI was associated with higher likelihood of kidney nonrecovery at discharge as well as higher Scr at discharge. Among those with no AKI, AKI stage 1, AKI stage 2, AKI stage 3 without KRT, and AKI-KRT, 1%, 7%, 11%, 41%, and 52% had a discharge Scr ≥ 1.5 times their baseline Scr or were continuing to receive KRT at discharge (Fig 3 ).
Figure 3.
Kidney outcomes of the ICU cohort, survivors only. AKI stage 1 defined as peak serum Cr is 1.5-1.9 times baseline serum Cr; AKI stage 2, peak serum Cr is 2.0-2.9 times baseline; AKI stage 3 without KRT, peak serum Cr is ≥3.0 times baseline. For clarity, bars less than 4% are unlabeled. Abbreviations: AKI, acute kidney injury; Cr, creatinine; ICU, intensive care unit; KRT, kidney replacement therapy.
Kidney Outcomes and Mortality in the AKI-KRT Subcohort
Among the 876 patients with AKI-KRT, the mean age was 61 ± 12 (SD) years, 626 (71.5%) were male, 362 (41.3%) were Black, and 177 (20.2%) were Hispanic or Latino. A minority (362 [41%]) had baseline eGFR ≤ 60 mL/min/1.73 m2. CKRT was the most common initial mode of therapy (n = 590 [67.4%]), and most patients (n = 665 [75.9%]) required at least 1 vasopressor/inotrope on the day of KRT initiation. Urine output was less than 500 mL/d in 521 patients (59.5%) and was less than 50 mL/d in 149 patients (17.0%) on KRT day 1. The median serum albumin was 2.5 (IQR, 2.1-2.8) g/dL, and the median arterial pH was 7.27 (IQR, 7.21-7.34). Before KRT initiation, 287 patients (32.8%) had received steroids, 155 (17.7%) received tocilizumab, and 54 (6.2%) received remdesivir. The median time elapsed from ICU admission to KRT day 1 was 3 (IQR, 1-6) days (Table 2 ).
Table 2.
Characteristics of the AKI-KRT Population, Stratified by Baseline eGFR
| Overall | Baseline eGFR, mL/min/1.73 m2 |
||||
|---|---|---|---|---|---|
| >60 | 31-60 | 16-30 | ≤15 | ||
| No. of patients | 876 (100%) | 514 (59%) | 210 (24%) | 89 (10%) | 63 (7%) |
| Age, y | 61 ± 12 | 59 ± 12 | 64 ± 12 | 63 ± 11 | 61 ± 15 |
| Male sex | 626 (71.5%) | 385 (74.9%) | 151 (71.9%) | 51 (57%) | 39 (62%) |
| Race | |||||
| White | 269 (30.7%) | 156 (30.4%) | 67 (31.9%) | 23 (26%) | 23 (37%) |
| Black or African American | 362 (41.3%) | 192 (37.4%) | 100 (47.6%) | 43 (48%) | 27 (43%) |
| Asian | 33 (3.8%) | 23 (4.5%) | 8 (3.8%) | 2 (2%) | 0 (0) |
| American Indian/Alaska Native | 7 (0.8%) | 4 (0.8%) | 3 (1.4%) | 0 (0) | 0 (0) |
| Native Hawaiian or Other Pacific Islander | 7 (0.8%) | 5 (1.0%) | 0 (0%) | 1 (1%) | 1 (2%) |
| More than 1 race | 8 (0.9%) | 7 (1.4%) | 1 (0.5%) | 0 (0%) | 0 (0%) |
| Unknown/not reported | 190 (21.7%) | 127 (24.7%) | 31 (14.8%) | 20 (23%) | 12 (19%) |
| Ethnicity | |||||
| Hispanic or Latino | 177 (20.2%) | 131 (25.5%) | 24 (11.4%) | 13 (15%) | 9 (14%) |
| Not Hispanic or Latino | 596 (68.0%) | 321 (62.5%) | 164 (78.1%) | 63 (71%) | 48 (76%) |
| Unknown | 103 (11.8%) | 62 (12.1%) | 22 (10.5%) | 13 (15%) | 6 (10%) |
| Diabetes mellitus | 471 (53.8%) | 248 (48.2%) | 127 (60.5%) | 61 (69%) | 35 (56%) |
| Initial mode of KRT | |||||
| CKRT 24 h/d | 471 (53.8%) | 278 (54.1%) | 115 (54.8%) | 48 (54%) | 30 (48%) |
| CKRT 12 h/d or less | 119 (13.6%) | 72 (14.0%) | 32 (15.2%) | 9 (10%) | 6 (10%) |
| Intermittent hemodialysis | 270 (30.8%) | 150 (29.2%) | 62 (29.5%) | 31 (35%) | 27 (43%) |
| Peritoneal dialysis | 8 (0.9%) | 7 (1.4%) | 0 (0.0) | 1 (1%) | 0 (0) |
| Unknown | 8 (0.9%) | 7 (1.4%) | 1 (0.5%) | 0 (0) | 0 (0) |
| Characteristics on KRT day 1 | |||||
| Serum albumin (g/dL) | 2.5 [2.1-2.8] | 2.49 [2.1-2.8] | 2.5 [2.1-2.9] | 2.4 [2.0-2.7] | 2.6 [2.2-3.1] |
| Arterial pH | 7.27 [7.21-7.34] | 7.27 [7.21-7.33] | 7.27 [7.20-7.33] | 7.28 [7.23-7.34] | 7.28 [7.21-7.36] |
| Urine output | |||||
| ≥500 mL/d | 266 (30.4%) | 154 (30.0%) | 71 (33.8%) | 25 (28%) | 16 (25%) |
| 50-499 mL/d | 372 (42.5%) | 229 (44.6%) | 88 (41.9%) | 35 (39%) | 20 (32%) |
| <50 mL/d | 149 (17.0%) | 87 (16.9%) | 29 (13.8%) | 19 (21%) | 14 (22%) |
| Unknown | 89 (10.2%) | 44 (8.6%) | 22 (10.5%) | 10 (11%) | 13 (21%) |
| No. of vasopressors/inotropes | |||||
| 0 | 211 (24.1%) | 119 (23.2%) | 48 (22.9%) | 25 (28%) | 19 (30%) |
| 1 | 400 (45.7%) | 233 (45.3%) | 96 (45.7%) | 39 (44%) | 32 (51%) |
| ≥2 | 265 (30.3%) | 162 (31.5%) | 66 (31.4%) | 25 (28%) | 12 (19%) |
| Major cardiac eventa | 55 (6.3%) | 37 (7.2%) | 10 (4.8%) | 5 (6%) | 3 (5%) |
| Medications administereda | |||||
| Corticosteroids | 287 (32.8%) | 169 (32.9%) | 82 (39.0%) | 20 (23%) | 16 (25%) |
| Tocilizumab | 155 (17.7%) | 99 (19.3%) | 40 (19.0%) | 10 (11%) | 6 (10%) |
| Remdesivir | 54 (6.2%) | 41 (8.0%) | 11 ( 5.2%) | 2 (2%) | 0 (0) |
| Mechanical ventilation | 840 (95.9%) | 502 (97.7%) | 202 (96.2%) | 77 (87%) | 59 (94%) |
| Platelets | |||||
| ≥150 ×103/μL | 703 (80.3%) | 415 (80.7%) | 174 (82.9%) | 65 (73%) | 49 (78%) |
| 100-149 ×103/μL | 106 (12.1%) | 62 (12.1%) | 23 (11.0%) | 15 (17%) | 6 (10%) |
| <100 ×103/μL | 61 (7.0%) | 36 (7.0%) | 12 (5.7%) | 7 (8%) | 6 (10%) |
| Unknown | 6 (0.7%) | 1 (0.2%) | 1 (0.5%) | 2 (2%) | 2 (3%) |
| Bilirubin | |||||
| <1.2 mg/dL | 594 (67.8%) | 317 (61.7%) | 153 (72.9%) | 72 (81%) | 52 (83%) |
| 1.2-1.9 mg/dL | 124 (14.2%) | 95 (18.5%) | 24 (11.4%) | 2 (2%) | 3 (5%) |
| ≥2.0+ mg/dL | 89 (10.2%) | 66 (12.8%) | 14 (6.7%) | 5 (6%) | 4 (6%) |
| Unknown | 69 (7.9%) | 36 (7.0%) | 19 (9.0%) | 10 (11%) | 4 (6%) |
| Days from ICU admission | 3 [1-6] | 4 [2-7] | 3.5 [2-6] | 2 [1-4] | 1 [0-2] |
Values for continuous variables given as mean ± SD or median [interquartile range]; for categorical data as frequency (%). Abbreviations: AKI, acute kidney injury; CKRT, continuous kidney replacement therapy; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; KRT, kidney replacement therapy.
Occurring on or before KRT day 1.
Discharge Status
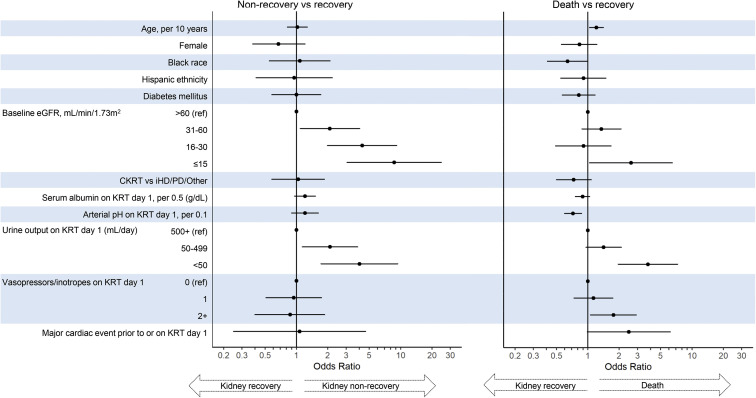
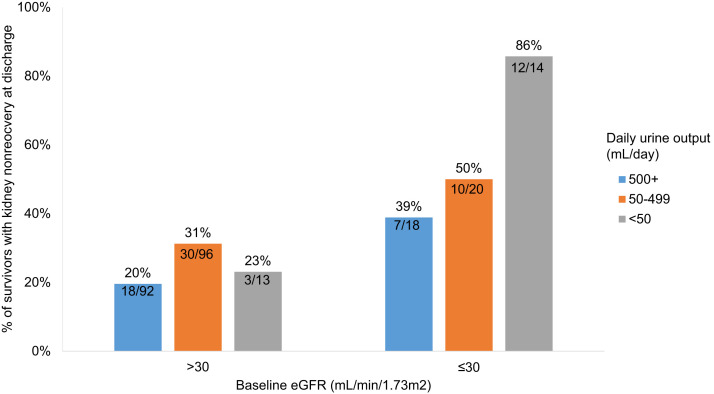
Of the 876 patients with AKI-KRT, 588 (67%) died, 95 (11%) were discharged alive and were continuing to receive dialysis at discharge, and 193 (22%) had kidney recovery by the time of discharge. In multinomial logistic regression models, lower baseline kidney function and lower urine output on KRT day 1 were each associated with kidney nonrecovery (Fig 4 ). The odds of nonrecovery approximately doubled with each more severe baseline eGFR category, with odds ratios of 2.09 (95% CI, 1.09-4.04), 4.27 (95% CI, 1.99-9.17), and 8.69 (95% CI, 3.07-24.55) for patients with eGFR of 31-60, 16-30, ≤15 mL/min/1.73 m2, respectively, compared with patients with eGFR > 60 mL/min/1.73 m2. Compared with patients with urine output ≥500 mL/d, oliguria (urine output 50-499 mL/d) was associated with a 2.10-fold increased odds of nonrecovery (95% CI, 1.14-3.88) and anuria (urine output <50 mL/d) was associated with a 4.02-fold increased odds of nonrecovery (95% CI, 1.72-9.39) (Fig 4; Table S1). A descriptive analysis of survivors also showed the association of both lower baseline eGFR and lower urine output with kidney nonrecovery (Fig 5 ). Of note, those who died had much shorter follow-up time (median, 7 [IQR, 3-15] days) compared with those who either had kidney recovery (31 [IQR, 23-44] days) or kidney nonrecovery (30 [IQR, 21-42.5] days), limiting interpretation of predictors of mortality.
Figure 4.
Multivariable multinomial regression, using recovery as the reference outcome. Abbreviations: CKRT, continuous kidney replacement therapy; eGFR, estimated glomerular filtration rate; iHD, intermittent hemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis.
Figure 5.
Percent of survivors with kidney nonrecovery at discharge, by baseline eGFR and urine output on KRT day 1. Each bar shows the percent with kidney nonrecovery at discharge, out of the survivors. For example, 92 patients with a baseline eGFR > 30 mL/min/1.73 m2 and urine output of ≥500 mL/d on KRT day 1 survived to discharge; 18 (20%) of these patients had kidney nonrecovery at discharge. Abbreviations: eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy.
Results were similar in secondary analyses using ordinal logistic regression: older age, baseline eGFR ≤ 15 mL/min/1.73 m2, lower arterial pH, and lower urine output were each associated with the composite outcome of nonrecovery or death, and older age, non-Black race, initiation with non-CKRT modality, lower albumin, lower arterial pH, anuria, need for 2 or more vasopressors/inotropes, and a preceding major cardiac event were each associated with mortality alone (Table S2). The cause-specific time-to-recovery analysis that treated death as a competing event identified Black race, baseline eGFR > 15 mL/min/1.73 m2, and greater urine output as associated with a higher likelihood of recovery (Table S3). Parsimonious and expanded analyses did not yield notably different results, though effect sizes were somewhat increased in parsimonious models (Tables S1-S3).
Kidney Function at Discharge Among Survivors
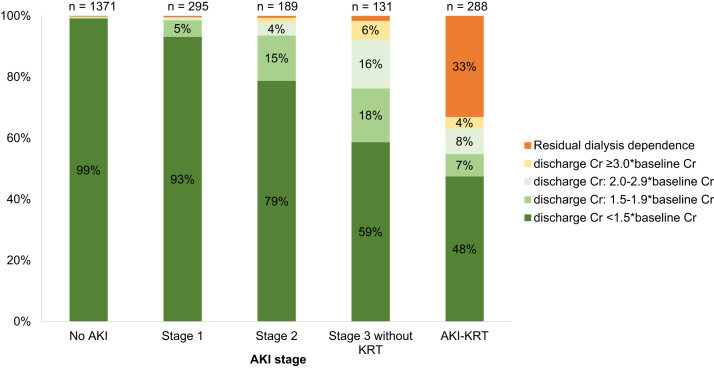
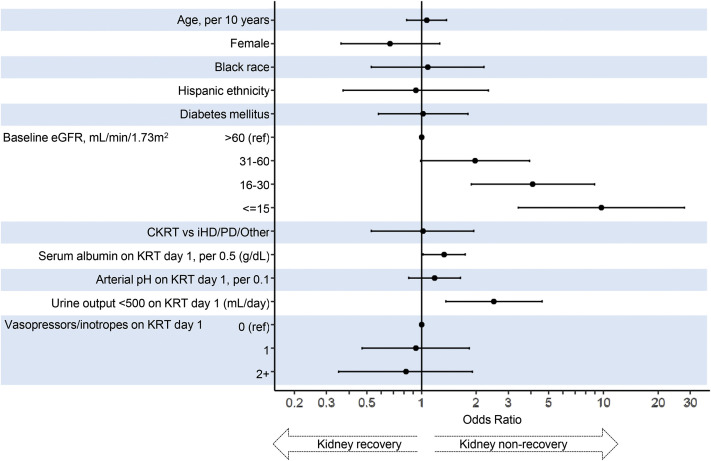
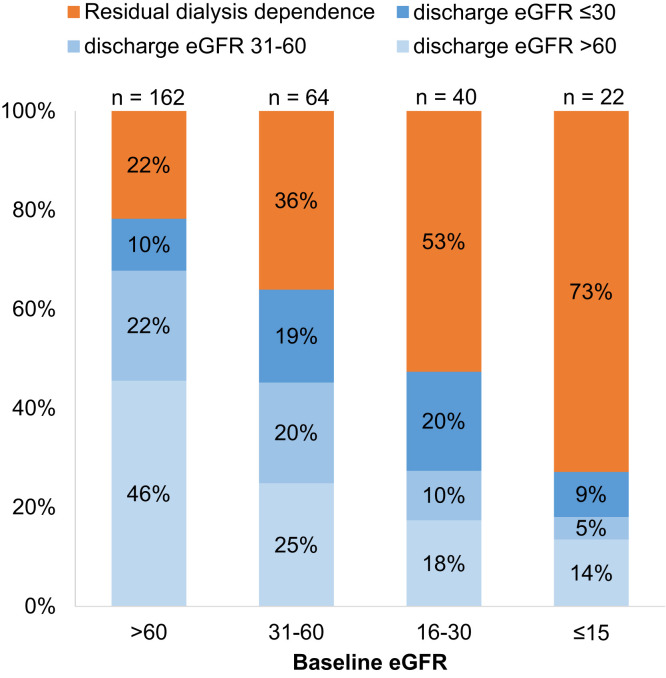
Among the 288 patients with AKI-KRT who survived to discharge, 162 (56.2%), 64 (22%), 40 (14%), and 22 (8%) had a baseline eGFR of >60, 31-60, 16-30, and ≤15 mL/min/1.73 m2, respectively. At discharge, 95 patients (33%) were continuing to receive dialysis. Lower baseline eGFR and oligoanuria (urine output <500 mL/d) on the day of KRT initiation were each associated with a higher likelihood of nonrecovery (Fig 6 ). Individuals with a higher baseline GFR were more likely to have recovery of kidney function to an eGFR of ≥60 mL/min/1.73 m2 at discharge (Fig 7 ).
Figure 6.
Outcome of kidney nonrecovery (vs recovery) at hospital discharge, among survivors. Abbreviations: CKRT, continuous kidney replacement therapy; eGFR, estimated glomerular filtration rate; iHD, intermittent hemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis.
Figure 7.
Kidney function at discharge among AKI-KRT patients, survivors only. Abbreviations: AKI-KRT, acute kidney injury treated with kidney replacement therapy; eGFR, estimated glomerular filtration rate.
Discussion
In this multicenter cohort study of 4,221 critically ill patients with COVID-19 admitted to ICUs at 68 US hospitals, we identified several noteworthy findings regarding outcomes in patients with AKI. First, greater AKI severity was associated with higher in-hospital mortality and, among survivors, worse kidney function at discharge. Second, among patients who had AKI and were treated with KRT, almost two-thirds died. Of those who survived to discharge, approximately two-thirds recovered kidney function and were not continuing to receive dialysis at discharge. Third, across multiple analyses, lower baseline eGFR and oligoanuria at dialysis initiation were each associated with lower likelihood of recovery from AKI-KRT.
Detailed study of kidney recovery among patients with COVID-19 with AKI-KRT has been limited. A single-center study in New York City from approximately the same time period as the current report found that, of 347 patients hospitalized with COVID-19 who developed AKI-KRT, only 87 (25%) survived to discharge1; among those who survived, 70% were not continuing to receive dialysis at discharge, findings that are comparable to our results. A small study in Berlin, Germany, from the same time period reported the outcomes of 74 patients with COVID-19 admitted to an ICU who developed AKI-KRT.16 Compared to our study, fewer patients had decreased eGFR at baseline, which may account for their higher rate of recovery, with only 3 of 34 survivors (8%) continuing to receive KRT at the end of follow-up. An earlier STOP-COVID study of patients enrolled through April 11, 2020, had found similar rates of in-hospital mortality and ongoing treatment with dialysis at discharge.
None of these studies have reported factors predictive of recovery from AKI-KRT. Our study expands on these data with a substantially larger cohort across multiple sites during the earliest wave of COVID-19 in the United States. The results of this report also expand on prior studies conducted with the STOP-COVID cohort by investigating associations of clinical factors at the time of dialysis initiation with kidney outcomes, including kidney recovery.8
Since this study was conducted, COVID-19 management has evolved to include the greater use of corticosteroids and other immunomodulatory agents (eg, tocilizumab) in patients with severe illness because these agents have been demonstrated to have a beneficial effect on patient survival.17 , 18 By contrast, our results showed steroids to be associated with greater mortality, perhaps indicating that during this phase they were selectively administered to those with more severe illness (Table S1). Interestingly, the same analysis found a nominal association between steroid use and kidney recovery, though it did not reach statistical significance; in a separate recent study, steroid use was associated with reduced risk for AKI progression.19 The observed association of steroid use with better kidney outcomes should be more systematically explored in subsequent larger clinical/epidemiologic investigations. Tocilizumab and remdesivir, which are also both now used more widely than during the study period, were not significantly associated with either kidney or mortality outcomes in this study.
Compared to prior studies of AKI-KRT among critically ill patients without COVID-19, this study and other studies of patients with COVID-19 have found higher rates of in-hospital mortality and of ongoing treatment with dialysis at hospital discharge.1 , 8 , 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 These differences in outcomes may reflect the profound multiorgan failure associated with COVID-19 during the initial spring 2020 surge. The higher rate of kidney nonrecovery may also be due to direct infection of the kidneys by the SARS-CoV-2 virus, though this remains controversial.29, 30, 31 Moreover, many of the studies of AKI among patients with COVID-19, including this study, have been conducted in academic hospitals, which tend to experience a higher acuity of disease. Nevertheless, despite variation in the rates of each outcome in the present study as compared with prior research in non–COVID-19 settings, the predictors of kidney recovery identified in the current study were similar to those found in studies among critically ill patients without COVID-19.
The association of both lower baseline eGFR and oliguria at the time of KRT initiation with a lower likelihood of kidney recovery highlights that kidney recovery after AKI is dependent on both the preinjury level of kidney function as well as the severity of the injury itself. Both elements of this conceptual model have been noted in other studies of recovery from AKI. Multiple studies have associated lower kidney function before an AKI episode with a higher likelihood of ongoing dialysis treatment or subsequent CKD progression.25 , 28 , 32 , 33 Of note, most of these studies were conducted in hospitalized patients without either a specified mechanism of AKI or a unifying underlying diagnosis, unlike this study that included only COVID-19 patients admitted to an ICU. Direct comparison of effect sizes is limited, however, by variation in outcome assessments. Other clinical predictors of kidney recovery have not been studied extensively. A prior prospective cohort study of ICU patients with AKI-KRT similarly noted an association between oliguria and ongoing treatment with dialysis, with kidney recovery assessed at 1 year.25
This work has considerable implications for clinical care, both during and after the COVID-19 pandemic. The magnitude of the effect sizes found in our models is striking. One may consider an example of comparing a patient with more than 500 mL/d of urine output at the time of dialysis initiation with a patient with anuria (less than 50 mL/d). Based on our findings, the odds of survival for the patient with normal urine output are 2.3 times higher than those of the patient with anuria, and his/her odds of kidney recovery are more than 4-fold increased. Compared with a patient with both anuria and eGFR of ≤15 mL/min/1.73 m2, a patient with neither condition has a 1.9-fold greater odds of survival and an almost 13-fold greater odds of kidney recovery. That a baseline eGFR ≤15 mL/min/1.73 m2 appears to confer some survival benefit may reflect earlier ICU admission and dialysis initiation for less severe illness in these patients with advanced CKD.
Accurate prognostication of outcomes can assist clinical decision-making when dialysis initiation is being considered for AKI. Nephrologists often use a “wait and see” approach in such cases; however, given the magnitude of effect sizes of factors such as reduced baseline eGFR and oliguria, one may reasonably assess the calculated odds and incorporate likelihood of kidney recovery and overall survival into conversations around goals of care. The Renal Physicians Association recommends shared decision-making in weighing the options of dialysis initiation, a time-limited trial of dialysis, or transition to end-of-life care.34 , 35 Given the high in-hospital mortality of patients with AKI-KRT and the impact of maintenance dialysis on quality of life, such conversations are critical to providing care consistent with patients’ goals and values.
This study has several strengths, particularly the detailed data collected by manual chart review in a large multicenter cohort of patients admitted to 68 ICUs around the United States, including acute severity of illness metrics obtained on the day of dialysis initiation. Although there is a heterogeneity of baseline burden of comorbid conditions, including baseline kidney function, an additional strength is a unifying nonsurgical, primary cause of acute illness—in this case, COVID-19—with a clear temporal pattern, thus reducing the heterogeneity present in many AKI-KRT studies. Although AKI due to COVID-19 may occur by multiple pathogenic mechanisms,12 it may be interesting to examine how well baseline eGFR and oliguria predict outcomes in other medical AKI populations.
We also acknowledge several limitations. First, Scr and KRT data were only collected for the first 14 days after ICU admission and on hospital discharge; as a result, outcomes were most reliably assessed at discharge. Logistic regression analysis was chosen to reflect this outcome assessment, but it assumes similar follow-up time among patients. Therefore, associations of exposures with mortality may have been biased by less follow-up time, though, given the overall short follow-up time for all patients, this bias is likely minimal, and similar results in time-to-event sensitivity analysis are reassuring. In addition, the uncertain timing of kidney recovery in some patients creates a bias toward the null in the time-to-recovery analysis. Furthermore, lack of postdischarge follow-up data precluded analysis of kidney recovery occurring after discharge, thus potentially underestimating kidney recovery rates. Reasons for KRT initiation were not captured. We were unable to determine how many deaths occurred after transfer to conservative management, which may partially account for the greater mortality rate observed among those with AKI stage 3 without KRT than those with AKI-KRT. Finally, addressing death as a competing outcome is always a challenge in studies of AKI-KRT outcomes, and the high mortality rate in this dataset likewise limits conclusions.
In summary, in this large cohort study of critically ill patients with COVID-19, decreased eGFR, and oliguria at the time of dialysis initiation were each significantly associated with a lower likelihood of kidney recovery. The magnitude of the associations presented here may assist prognostication of long-term dialysis treatment, which carries implications for patients’ physical health and quality of life.
Article Information
STOP-COVID Investigators
In addition to authors Hsu, Gupta,∗ Goyal,∗ Faugno, Tariq, Raichoudhury,∗ Sharma, Meyer, Kshirsagar, Leaf,∗ and Weiner, the STOP-COVID Investigators are as follows (∗Site Principal Investigator). Baylor College of Medicine: Carl P. Walther∗ and Samaya J. Anumudu; Baylor University Medical Center: Justin Arunthamakun,∗ Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, and Thuy-Duyen Nguyen; Beth Israel Deaconess Medical Center: Shahzad Shaefi,∗ Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, Juan D. Valencia, and Kenneth A. Bauer; Boston Medical Center: Sushrut S. Waikar∗ and Zoe A. Kibbelaar; Cook County Health: Ambarish M. Athavale,∗ Peter Hart, Shristi Upadhyay, Ishaan Vohra, and Ajiboye Oyintayo; Cooper University Health Care: Adam Green,∗ Jean-Sebastien Rachoin, Christa A. Schorr, and Lisa Shea; Duke University Medical Center: Daniel L. Edmonston∗ and Christopher L. Mosher; Hackensack Meridian Health Mountainside Medical Center: Alexandre M. Shehata,∗ Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, and Aquino Williams; Hackensack Meridian Health Hackensack University Medical Center: Samantha K. Brenner,∗ Patricia Walters, Ronaldo C. Go, and Keith M. Rose; Harvard T.H. Chan School of Public Health: Miguel A. Hernán; Harvard University: Amy M. Zhou, Ethan C. Kim, and Rebecca Lisk; Icahn School of Medicine at Mount Sinai: Lili Chan,∗ Kusum S. Mathews,∗ Steven G. Coca, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Pattharawin Pattharanitima, and Emily J. Gallagher; Indiana University School of Medicine/Indiana University Health: Allon N. Friedman,∗ John Guirguis, Rajat Kapoor, Christopher Meshberger, and Katherine J. Kelly; Johns Hopkins Hospital: Chirag R. Parikh,∗ Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Carmen Elena Cervantes, and Samir C. Gautam; Kings County Hospital Center: Mary C. Mallappallil,∗ Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddhartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, and Leon Boudourakis; Loma Linda University: H. Bryant Nguyen∗ and Afshin Ahoubim; Mayo Clinic, Arizona: Leslie F. Thomas∗ and Dheeraj Reddy Sirganagari; Mayo Clinic, Florida: Pramod K. Guru∗; Mayo Clinic, Rochester: Kianoush Kashani∗ and Shahrzad Tehranian; Medical College of Wisconsin: Yan Zhou,∗ Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, and Mrigank S. Gupta; MedStar Georgetown University Hospital: Princy N. Kumar,∗ Deepa G. Lazarous, and Seble G. Kassaye; Montefiore Medical Center/Albert Einstein College of Medicine: Michal L. Melamed,∗ Tanya S. Johns. Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Jyotsana Thakkar, Neelja Kumar, Michael J. Ross, and Michael Chang; New York-Presbyterian Queens Hospital: Akshay Athreya and Mohamed Farag; New York-Presbyterian/Weill Cornell Medical Center: Edward J. Schenck,∗ Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, and William Whalen; New York University Langone Hospital: David Charytan∗ and Ashley Macina; Northwestern Memorial Hospital, Northwestern University Feinberg School of Medicine: Anand Srivastava,∗ Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, and Alexander J. Hodakowski; Ochsner Medical Center: Juan Carlos Q. Velez,∗ Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, and Muner M.B. Mohamed; Oregon Health and Science University Hospital: Rupali S. Avasare∗ and David Zonies∗; Partners Healthcare, Brigham and Women’s Hospital, Brigham and Women’s Faulkner Hospital, Massachusetts General Hospital, and Newton Wellesley Hospital: Hanny Al-Samkari, Rebecca Karp Leaf, Rachel Rosovsky, Meghan E. Sise, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Shreyak Sharma, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Omer Kamal, Wei Wang, Meghan Lee, Ian A. Strohbehn, Jiahua Li, and Ariel L. Mueller; ProMedica Health System: Roberta E. Redfern,∗ Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, and Laura Bickley; Renown Health: Chris Rowan∗ and Farah Madhani-Lovely∗; Rush University Medical Center: Vasil Peev,∗ Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, and Joy-Marie Hermes; Rutgers/New Jersey Medical School: Anne K. Sutherland,∗ Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, and Mark Liotta; Rutgers/Robert Wood Johnson Medical School: Jared Radbel,∗ Sonika Puri, Jag Sunderram, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim, Jayanth S. Vatson, and George Karp; Stanford Healthcare, Stanford University School of Medicine: Shuchi Anand,∗ Joseph E. Levitt, and Pablo Garcia; Temple University Hospital: Suzanne M. Boyle∗ and Rui Song; Thomas Jefferson University Hospital: Jingjing Zhang,∗ Sang Hoon Woo, Xiaoying Deng, and Goni Katz-Greenberg; Tulane Medical Center: Moh’d A. Sharshir∗ and Vadym V. Rusnak; United Health Services Hospitals: Muhammad Imran Ali; University of Colorado Anschutz Medical Campus: Anip Bansal,∗ Amber S. Podoll, Michel Chonchol, Sunita Sharma, and Ellen L. Burnham; University Hospitals Cleveland Medical Center: Arash Rashidi∗ and Rana Hejal; University of Alabama-Birmingham Hospital: Eric Judd,∗ Laura Latta, and Ashita Tolwani; University of California-Davis Medical Center: Timothy E. Albertson∗ and Jason Y. Adams; University of California-Los Angeles Medical Center, Ronald Reagan-UCLA Medical Center: Steven Y. Chang∗ and Rebecca M. Beutler; Santa Monica-UCLA Medical Center: Carl E. Schulze; University of California-San Diego Medical Center: Etienne Macedo∗ and Harin Rhee; University of California-San Francisco Medical Center: Kathleen D. Liu∗ and Vasantha K. Jotwani; University of Chicago Medical Center: Jay L. Koyner∗; University of Florida Health-Gainesville: Chintan V. Shah∗; University of Florida-Health-Jacksonville: Vishal Jaikaransingh∗; University of Illinois Hospital and Health Sciences System: Stephanie M. Toth-Manikowski,∗ Min J. Joo,∗ and James P. Lash; University of Kentucky Medical Center: Javier A. Neyra,∗ Nourhan Chaaban, Madona Elias, and Yahya Ahmad; University Medical Center of Southern Nevada: Rajany Dy,∗ Alfredo Iardino, and Elizabeth H. Au; University of Miami Health System: Marie Anne Sosa,∗ Sabrina Taldone, Gabriel Contreras, David De La Zerda, Hayley B. Gershengorn, and Alessia Fornoni; University of Michigan: Salim S. Hayek,∗ Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’Hayer, Chelsea Meloche, Rafey Feroze, Kishan J. Padalia, Jeff Leya, John P. Donnelly, Andrew J. Admon; University of North Carolina School of Medicine: Jennifer E. Flythe,∗ Matthew J. Tugman, and Emily H. Chang; University of Oklahoma Health Sciences Center: Brent R. Brown∗; University of Pennsylvania Health System: Amanda K. Leonberg-Yoo,∗ Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, and Charles R. Vasquez; University of Pittsburgh Medical Center: Amar D. Bansal,∗ Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, and Huiwen Chen; University of Tennessee Health Science Center and Memphis VA Medical Center/Methodist University Hospital: Csaba P. Kovesdy,∗ Miklos Z. Molnar,∗ and Ambreen Azhar; University of Texas Southwestern Medical Center and Parkland Health and Hospital System: S. Susan Hedayati,∗ Mridula V. Nadamuni, Shani Shastri, and Duwayne L. Willett; University of Vermont Larner College of Medicine: Samuel A.P. Short; University of Virginia Health System: Amanda D. Renaghan∗ and Kyle B. Enfield; University of Washington Medical Center: Pavan K. Bhatraju∗ and A. Bilal Malik; Vanderbilt University Medical Center: Matthew W. Semler; Washington University in St. Louis/Barnes Jewish Hospital: Anitha Vijayan,∗ Christina Mariyam Joy, Tingting Li, Seth Goldberg, and Patricia F. Kao; Wellforce Health System, Lowell General Hospital: Greg L. Schumaker∗; Tufts Medical Center: Greg L. Schumaker; Westchester Medical Center: Marta Christov,∗ Jennifer Griffiths, Sanjeev Gupta, Aromma Kapoor, and Savneek Chugh; and Yale School of Medicine: Perry Wilson,∗ Tanima Arora, and Ugochukwu Ugwuowo.
Authors’ Full Names and Academic Degrees
Caroline M. Hsu, MD, Shruti Gupta, MD, MPH, Hocine Tighiouart, MS, Nitender Goyal, MD, Anthony J. Faugno, MD, Asma Tariq, MD, Ritesh Raichoudhury, MD, Jill H. Sharma, MD, Leah Meyer, BA, Ravi K. Kshirsagar, MD, Aju Jose, MBBS, David E. Leaf, MD, MMSc, and Daniel E. Weiner, MD, MS.
Authors’ Contributions
Research idea and study design: CMH & SG (equal contribution), HT, NG, DEL & DEW (equal contribution); data acquisition: CMH & SG (equal contribution), HT, NG, AJF, AT, RR, JHS, LM, RKK, AJ, DEL & DEW (equal contribution); data analysis/interpretation: CMH & SG (equal contribution), HT, DEL & DEW (equal contribution); statistical analysis: CMH, HT; supervision or mentorship: SG, HT, NG, DEL & DEW (equal contribution). Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
This work was supported by the American Society of Nephrology KidneyCure Foundation’s Ben J. Lipps Research Fellowship and NIH grant T32 DK007777 (to Dr Hsu), and NIH grants R01HL144566 and R01DK125786 (to Dr Leaf). These funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Financial Disclosure
Dr Gupta has received funding from GE Healthcare and is a scientific coordinator for GlaxoSmithKline’s ASCEND study. The remaining authors declare that they have no other relevant financial interests.
Peer Review
Received August 16, 2021. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form November 22, 2021.
Footnotes
Complete author and article information (including a list of STOP-COVID Investigators) provided before references.
Item S1: Details of analytical methods for the AKI-KRT subcohort.
Table S1: Multinomial logistic regression model, with discharge without KRT as the reference.
Table S2: Ordinal logistic regression model.
Table S3: Cause-specific hazard model.
Contributor Information
STOP-COVID Investigators:
Hsu Gupta, Goyal Faugno, Tariq Raichoudhury, Sharma Meyer, Kshirsagar Leaf, Carl P. Walther, Samaya J. Anumudu, Justin Arunthamakun, Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, Thuy-Duyen Nguyen, Shahzad Shaefi, Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, Juan D. Valencia, Kenneth A. Bauer, Sushrut S. Waikar, Zoe A. Kibbelaar, Ambarish M. Athavale, Peter Hart, Shristi Upadhyay, Ishaan Vohra, Ajiboye Oyintayo, Adam Green, Jean-Sebastien Rachoin, Christa A. Schorr, Lisa Shea, Daniel L. Edmonston, Christopher L. Mosher, Alexandre M. Shehata, Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, Aquino WilliamsSamantha K. Brenner, Patricia Walters, Ronaldo C. Go, Keith M. Rose, Miguel A. Hernán, Amy M. Zhou, Ethan C. Kim, Rebecca Lisk, Lili Chan, Kusum S. Mathews, Steven G. Coca, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Pattharawin Pattharanitima, Emily J. Gallagher, Allon N. Friedman, John Guirguis, Rajat Kapoor, Christopher Meshberger, Katherine J. Kelly, Chirag R. Parikh, Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Carmen Elena Cervantes, Samir C. Gautam, Mary C. Mallappallil, Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddhartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, Leon Boudourakis, H. Bryant Nguyen, Afshin Ahoubim, Leslie F. Thomas, Dheeraj Reddy Sirganagari, Pramod K. Guru, Kianoush Kashani, Shahrzad Tehranian, Yan Zhou, Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, Mrigank S. Gupta, Princy N. Kumar, Deepa G. Lazarous, Seble G. Kassaye, Michal L. Melamed, Tanya S. Johns. Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Jyotsana Thakkar, Neelja Kumar, Michael J. Ross, Michael Chang, Akshay Athreya, Mohamed Farag, Edward J. Schenck, Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, William Whalen, David Charytan, Ashley Macina, Anand Srivastava, Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, Alexander J. Hodakowski, Juan Carlos Q. Velez, Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, Muner M.B. Mohamed, Rupali S. Avasare, David Zonies, Hanny Al-Samkari, Rebecca Karp Leaf, Rachel Rosovsky, Meghan E. Sise, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Shreyak Sharma, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Omer Kamal, Wei Wang, Meghan Lee, Ian A. Strohbehn, Jiahua Li, Ariel L. Mueller, Roberta E. Redfern, Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, Laura Bickley, Chris Rowan, Farah Madhani-Lovely, Vasil Peev, Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, Joy-Marie Hermes, Anne K. Sutherland, Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, Mark Liotta, Jared Radbel, Sonika Puri, Jag Sunderram, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim, Jayanth S. Vatson, George Karp, Shuchi Anand, Joseph E. Levitt, Pablo Garcia, Suzanne M. Boyle, Rui Song, Jingjing Zhang, Sang Hoon Woo, Xiaoying Deng, Goni Katz-Greenberg, Moh’d A. Sharshir, Vadym V. Rusnak, Anip Bansal, Amber S. Podoll, Michel Chonchol, Sunita Sharma, Ellen L. Burnham, Arash Rashidi, Rana Hejal, Eric Judd, Laura Latta, Ashita Tolwani, Timothy E. Albertson, Jason Y. Adams, Steven Y. Chang, Rebecca M. Beutler, Carl E. Schulze, Etienne Macedo, Harin RheeKa, thleen D. Liu, Vasantha K. Jotwani, Jay L. Koyner, Chintan V. Shah, Vishal Jaikaransingh, Stephanie M. Toth-Manikowski, Min J. Joo, James P. Lash, Javier A. Neyra, Nourhan Chaaban, Madona Elias, Yahya Ahmad, Rajany Dy, Alfredo Iardino, Elizabeth H. Au, Marie Anne Sosa, Sabrina Taldone, Gabriel Contreras, David De La Zerda, Hayley B. Gershengorn, Alessia Fornoni, Salim S. Hayek, Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’Hayer, Chelsea Meloche, Rafey Feroze, Kishan J. Padalia, Jeff Leya, John P. Donnelly, Andrew J. Admon, Jennifer E. Flythe, Matthew J. Tugman, Emily H. Chang, Brent R. Brown, Amanda K. Leonberg-Yoo, Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, Charles R. Vasquez, Amar D. Bansal, Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, Huiwen Chen, Csaba P. Kovesdy, Miklos Z. Molnar, Ambreen Azhar, S. Susan Hedayati, Mridula V. Nadamuni, Shani Shastri, Duwayne L. Willett, Samuel A.P. Short, Amanda D. Renaghan, Kyle B. Enfield, Pavan K. Bhatraju, A. Bilal Malik, Matthew W. Semler, Anitha Vijayan, Christina Mariyam Joy, Tingting Li, Seth Goldberg, Patricia F. Kao, Greg L. Schumaker, Marta Christov, Jennifer Griffiths, Sanjeev Gupta, Aromma Kapoor, Savneek Chugh, Perry Wilson, Tanima Arora, and Ugochukwu Ugwuowo
Supplementary Material
Item S1. Tables S1-S3.
References
- 1.Chan L., Chaudhary K., Saha A., et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver S.A., Beaubien-Souligny W., Shah P.S., et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Med. 2021;3(1):83–98.e1. doi: 10.1016/j.xkme.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins-Juarez S.Y., Qian L., King K.L., et al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020;5(8):1149–1160. doi: 10.1016/j.ekir.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen K.L., Chertow G.M., Foley R.N., et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2021;77(4):A7–A8. doi: 10.1053/j.ajkd.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus M.A., Fluck R.J., Weinhandl E.D., et al. Intensive hemodialysis and health-related quality of life. Am J Kidney Dis. 2016;68(5S1):S33–S42. doi: 10.1053/j.ajkd.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Flythe J.E., Hilliard T., Castillo G., et al. Symptom prioritization among adults receiving in-center hemodialysis: a mixed methods study. Clin J Am Soc Nephrol. 2018;13(5):735–745. doi: 10.2215/CJN.10850917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S., Coca S.G., Chan L., et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2021;32(1):161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B.J., Hsu C.-Y., Parikh R.V., et al. Non-recovery from dialysis-requiring acute kidney injury and short-term mortality and cardiovascular risk: a cohort study. BMC Nephrol. 2018;19(1):134. doi: 10.1186/s12882-018-0924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah S., Leonard A.C., Harrison K., Meganathan K., Christianson A.L., Thakar C.V. Mortality and recovery associated with kidney failure due to acute kidney injury. Clin J Am Soc Nephrol. 2020;15(7):995–1006. doi: 10.2215/CJN.11200919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellum J.A., Sileanu F.E., Bihorac A., Hoste E.A.J., Chawla L.S. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadim M.K., Forni L.G., Mehta R.L., et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S., Hayek S.S., Wang W., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 15.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockmann H., Hardenberg J.-H.B., Aigner A., et al. High rates of long-term renal recovery in survivors of coronavirus disease 2019-associated acute kidney injury requiring kidney replacement therapy. Kidney Int. 2021;99(4):1021–1022. doi: 10.1016/j.kint.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne J.A.C., Murthy S., et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siemieniuk R.A., Bartoszko J.J., Ge L., et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumlertgul N., Pirondini L., Cooney E., et al. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11(1):123. doi: 10.1186/s13613-021-00914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sindhu C, Prasad P, Elumalai R, Matcha J. Clinical profile and outcomes of COVID-19 patients with acute kidney injury: a tertiary centre experience from South India. Clin Exp Nephrol. Published online August 16, 2021. https://doi.org/10.1007/s10157-021-02123-7 [DOI] [PMC free article] [PubMed]
- 21.Ng J.H., Hirsch J.S., Hazzan A., et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77(2):204–215.e1. doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charytan D.M., Parnia S., Khatri M., et al. Decreasing incidence of acute kidney injury in patients with COVID-19 critical illness in New York City. Kidney Int Rep. 2021;6(4):916–927. doi: 10.1016/j.ekir.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowe B., Cai M., Xie Y., Gibson A.K., Maddukuri G., Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnassieux M., Duclos A., Schneider A.G., et al. Renal replacement therapy modality in the ICU and renal recovery at hospital discharge. Crit Care Med. 2018;46(2):e102–e110. doi: 10.1097/CCM.0000000000002796. [DOI] [PubMed] [Google Scholar]
- 25.De Corte W., Dhondt A., Vanholder R., et al. Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: a prospective cohort study. Crit Care. 2016;20(1):256. doi: 10.1186/s13054-016-1409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiffl H., Fischer R. Five-year outcomes of severe acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant. 2008;23(7):2235–2241. doi: 10.1093/ndt/gfn182. [DOI] [PubMed] [Google Scholar]
- 27.Triverio P.-A., Martin P.-Y., Romand J., Pugin J., Perneger T., Saudan P. Long-term prognosis after acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant. 2009;24(7):2186–2189. doi: 10.1093/ndt/gfp072. [DOI] [PubMed] [Google Scholar]
- 28.Hsu C., Chertow G.M., McCulloch C.E., Fan D., Ordoñez J.D., Go A.S. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moledina D.G., Simonov M., Yamamoto Y., et al. The association of COVID-19 with acute kidney injury independent of severity of illness: a multicenter cohort study. Am J Kidney Dis. 2021;77(4):490–499.e1. doi: 10.1053/j.ajkd.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassler L., Reyes F., Sparks M., Welling P., Batlle D. Evidence for and against direct kidney infection by SARS-CoV-2 in patients with COVID-19. Clin J Am Soc Nephrol. 2021;16(11):1755–1765. doi: 10.2215/CJN.04560421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan S., Chen L., Yang C.-R., Raghuram V., Khundmiri S.J., Knepper M.A. Does SARS-CoV-2 infect the kidney? J Am Soc Nephrol. 2020;31(12):2746–2748. doi: 10.1681/ASN.2020081229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimes-Stigare C., Frumento P., Bottai M., Mårtensson J., Martling C.-R., Bell M. Long-term mortality and risk factors for development of end-stage renal disease in critically ill patients with and without chronic kidney disease. Crit Care. 2015;19:383. doi: 10.1186/s13054-015-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James M.T., Pannu N., Hemmelgarn B.R., et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA. 2017;318(18):1787–1797. doi: 10.1001/jama.2017.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renal Physicians Association Shared Decision-Making in the Appropriate Initiation of and Withdrawal From Dialysis: Clinical Practice Guideline. 2nd ed. Renal Physicians Association; 2010. https://cdn.ymaws.com/www.renalmd.org/resource/resmgr/Store/Shared_Decision_Making_Recom.pdf
- 35.Scherer J.S., Holley J.L. The role of time-limited trials in dialysis decision making in critically ill patients. Clin J Am Soc Nephrol. 2016;11(2):344–353. doi: 10.2215/CJN.03550315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1. Tables S1-S3.