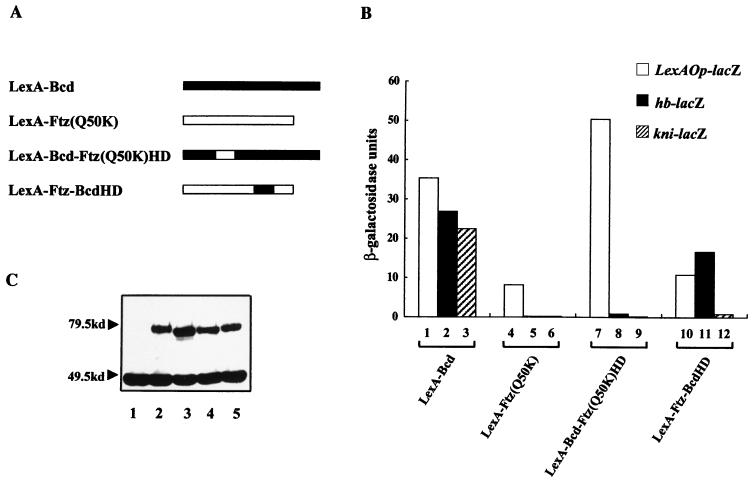
FIG. 2.
Chimeric proteins assayed in yeast cells reveal important functions of Bcd. (A) Schematic diagram of the activators used in our study. The exchanged homeodomains in the two hybrid molecules are shown as open and solid boxes. The DNA binding domain of LexA is not shown in this diagram because it is present in all of the proteins. (B) Activation assay results. Different activator proteins were assayed for their abilities to activate transcription from the integrated reporter genes hb-lacZ, kni-lacZ, and LexAOp-lacZ. Shown are β-galactosidase activities obtained from these assays. (C) Western blot assay using antibodies against LexA. Lanes 1 to 5 represent results using yeast cell lysates containing no activator, LexA-Ftz(Q50K), LexA-Bcd, LexA-Bcd-Ftz(Q50K)HD, and LexA-Ftz-BcdHD, respectively. A nonspecific band at ∼46 kDa, which has been reported previously (46), can be used as an internal control.