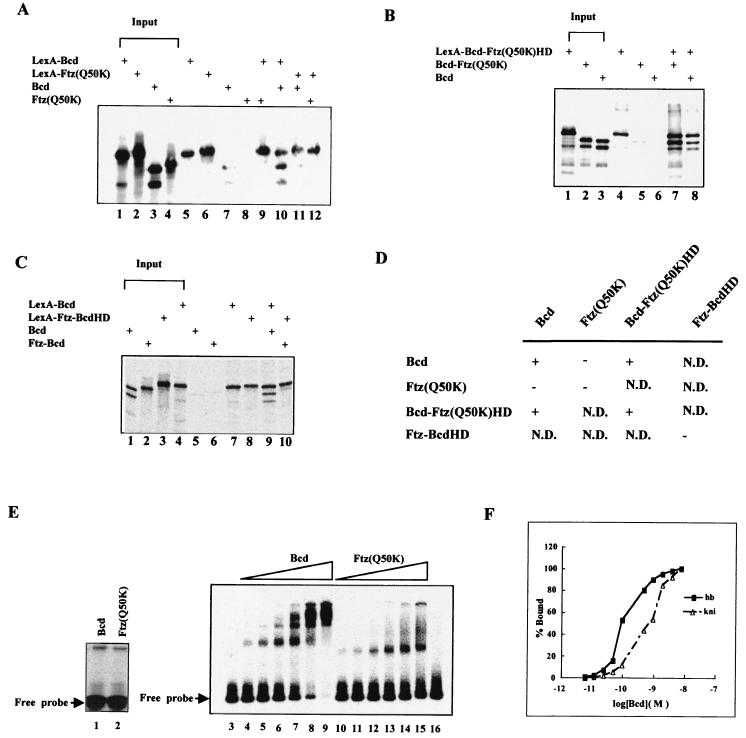
FIG. 3.
Bcd protein sequences outside the homeodomain participate in protein-protein interaction. (A to C) Shown are coimmunoprecipitation assay results. In vitro-generated and radioactively labeled proteins were incubated and then precipitated with antibodies against LexA (see Materials and Methods for details). The input represents one-fifth of the amount of proteins used in the coimmunoprecipitation assays. +, present. (D) Summary of coimmunoprecipitation assay results. +, interaction; −, no interaction; N.D., not determined. (E) Gel shift assays using in vitro-translated full-length Bcd and Ftz(Q50K). The experiments shown in lanes 3 to 6 used a modified hb enhancer element (∼1.5 × 10−11 M) with six consensus sites (Fig. 6A). Lanes 3 and 16 represent experiments with no translation lysate and 10 μl of luciferase translation lysate added, respectively. The experiments in lanes 1 and 2 were performed on a single-Bcd-site probe (∼10−8 M), permitting an estimate of the amounts of the active proteins (1 μl of translation lysates used). According to this estimate, similar amounts of active proteins were used in the experiments shown in lanes 4 to 15: 0.25, 0.5, 1, 2, 4, and 8 μl of Bcd translation lysates for lanes 4 to 9, respectively; 0.3, 0.6, 1.25, 2.5, 5, and 10 μl of Ftz(Q50K) translation lysates for lanes 10 to 15, respectively. (F) Residual cooperativity of Bcd homeodomain on hb and kni enhancer elements. Shown are binding curves of the recombinant Bcd homeodomain on the hb and kni enhancer elements measured in a gel shift assay. The occupancy of the Bcd homeodomain on DNA sites was calculated as follows: the sum of the amount of shifted complex × number of protein molecules in the complex/total number of Bcd sites on the probe (8). % Bound, fraction of maximal binding. The results in this figure are consistent with those published previously (8). The cooperativity provided by the homeodomain is very modest compared to that of full-length Bcd: it takes about a 70-fold increase in Bcd homeodomain concentration to achieve from 5 to 95% binding to the hb enhancer element, in contrast to less than a 4-fold increase for full-length Bcd to achieve similar binding (37, 63) (see panel E).