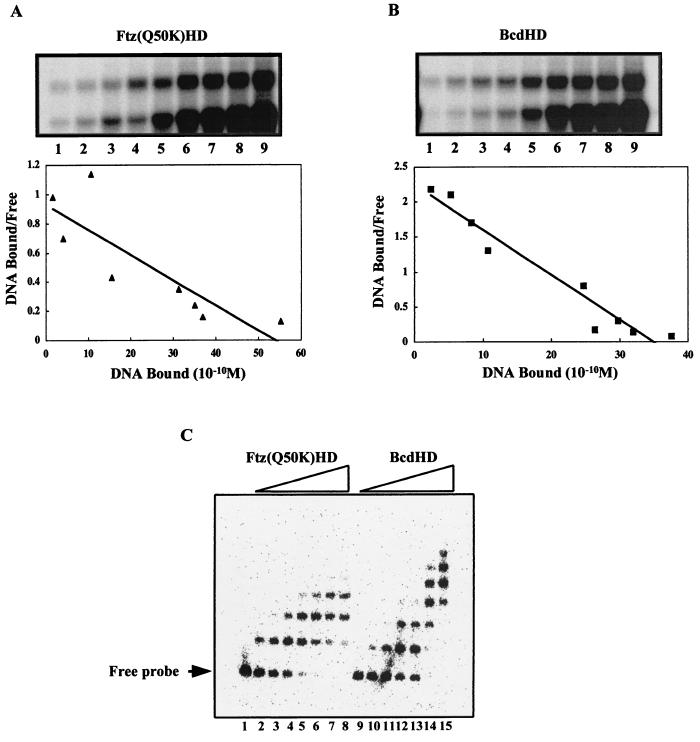
FIG. 4.
Bcd and Ftz(Q50K) homeodomains have comparable affinities to a consensus Bcd site but bind to the kni enhancer element differently. (A and B) Gel shift assays for a Scatchard analysis to determine the affinities of recombinant Bcd and Ftz(Q50K) homeodomains for the consensus site A1. The DNA concentrations used in these analyses were 6 × 10−11, 1.2 × 10−10, 1.8 × 10−10, 2.4 × 10−10, 6.0 × 10−10, 1.2 × 10−9, 1.8 × 10−9, 2.4 × 10−9, and 4.8 × 10−9 M for lanes 1 to 9, respectively. The Kd values given in the text represent averages of results of three independent assays. (C) Results of gel shift assays of recombinant Bcd and Ftz(Q50K) homeodomains on the natural kni enhancer element. At low concentrations, the bindings of these proteins appear similar (lanes 2 to 3 and 10 to 11). However, at higher concentrations of the proteins, complexes containing more protein molecules were obtained for the Bcd homeodomain than for the Ftz(Q50K) homeodomain (lanes 7 to 8 and 14 to 15). See the text for further details. Since the kni enhancer element contains all nonconsensus sites, we do not know exactly which of them is recognized by the Ftz(Q50K) homeodomain. Two potential candidate sites in the kni enhancer element deviate from the consensus site only modestly because they still contain a TAAT core: TAATCG and TAATCT. The Bcd homeodomain exhibits little cooperativity on the kni enhancer element (Fig. 3F); therefore, the protein-DNA complexes observed here represent primarily progressive independent recognition of different Bcd sites.