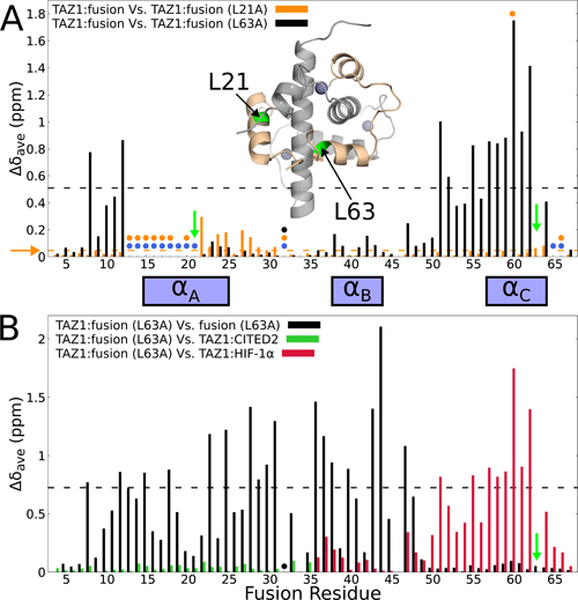
Figure 6. Site-directed mutagenesis to probe TAZ1-mediated allosteric interactions between the fusion peptide αA and αC helices.
(A) Weighted average 1H,15N chemical shift differences between the original 15N-labeled fusion peptide in complex with unlabeled TAZ1 and 15N-labeled L21A (orange) or 15N-labeled L63A (black) fusion peptide in complex with unlabeled TAZ1. Green arrows indicate the sites of mutation. Circles denote residues for which backbone amide cross peaks are broadened beyond detection for the TAZ1 complexes of the original fusion peptide (blue), L21A fusion peptide (orange), and L63A fusion peptide (black). Residues for which there are no circles and no data are prolines. The broken horizontal lines (and horizontal orange arrow) denote one standard deviation above the 10 % trimmed mean for the TAZ1:fusion vs. TAZ1:fusion (L21A) (orange) and TAZ1:fusion vs. TAZ1:fusion (L63A) (black) data sets. Inset: structure of the fusion peptide complex, with TAZ1 shown in gray and the fusion peptide shown in tan. Residues L21 and L63 are labeled and colored green. Zn atoms are shown as gray spheres. (B) Weighted average 1H,15N chemical shift differences for the TAZ1-bound L63A fusion peptide relative to the unbound state (black), to residues 220–245 of CITED in complex with TAZ1 (green, corresponding to residues 10–35 of the fusion peptide), and to residues 795–826 of HIF-1α in complex with TAZ1 (red, corresponding to residues 36–67 of the fusion peptide).The green arrow indicates the site of mutation. The backbone amide resonance corresponding to E32 is broadened beyond detection and is marked with a black circle. The broken black line denotes one standard deviation above the 10 % trimmed mean of the TAZ1:fusion (L63A) vs. free L63A fusion peptide shift differences. The boxes between panels A and B denote the positions of the CITED2 αA and HIF-1α αB and αC binding motifs.