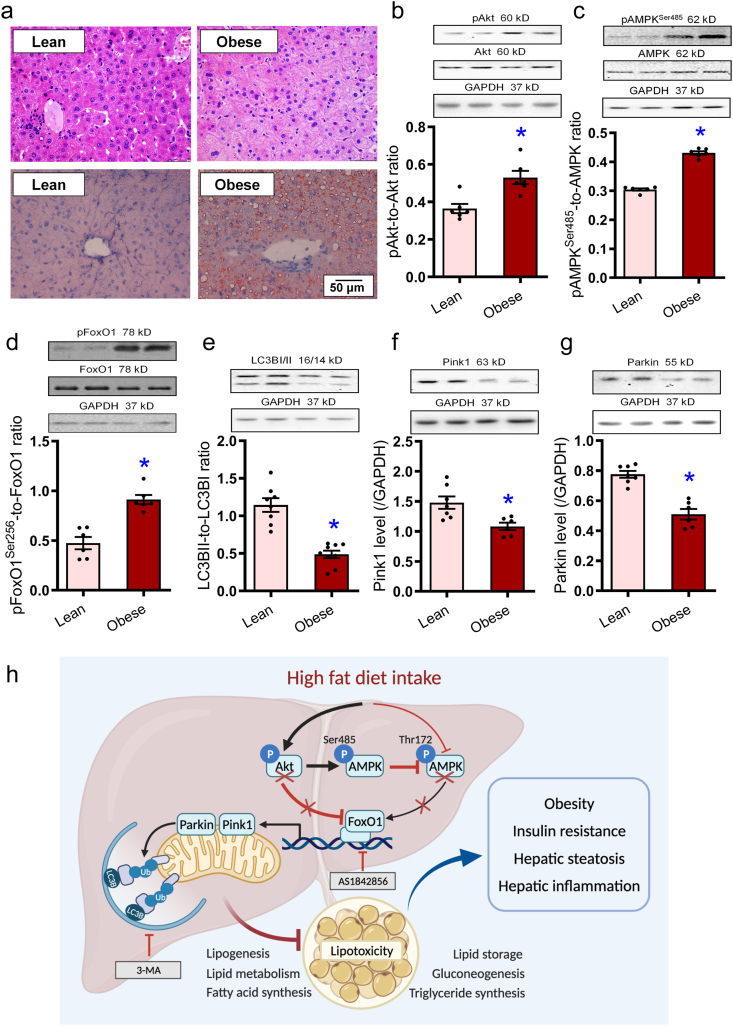
Figure 11.
Morphology, steatosis, cell signaling, autophagy, and Pink1–Parkin mitophagy in obese human liver specimen. (a) H&E staining of liver cross-sections and oil red O staining micrographs in lean and obese human liver samples. (b) pAkt-to-Akt ratio. (c) pAMPK (Ser485)-to-AMPK ratio. (d) pFoxO1-to-FoxO1 ratio. (e) LC3BII-to-LC3BI ratio. (f) Pink1. (g) Parkin. Representative gel blots depicting pan- and phosphorylated Akt, AMPK (Ser485), and FoxO1, as well as LC3BI/II, Pink1, and Parkin (GAPDH as loading controls) using specific antibodies were shown. Data are expressed as mean ± SEM (n = 6–7). ∗P < 0.05 vs. lean group. h: Scheme depicting possible mechanism(s) for the role of Akt2, AMPK, and Parkin-mediated mitophagy in high fat diet-induced obesity and hepatic steatosis. Chronic high fat diet intake suppresses FoxO1-mediated mitophagy through parallel regulation of Akt and AMPK. On one hand, high fat intake promotes Akt phosphorylation leading to inhibition of FoxO1 (phosphorylation) and suppressed autophagy. On the other hand, high fat intake suppressed AMPK phosphorylation at Thr172 and inhibition of autophagy. Hyperactivated Akt (phosphorylation) fosters AMPK phosphorylation at Ser485, disturbing AMPK phosphorylation at Thr172 indirectly. Compromised FoxO1 signaling contributes to compromised lipid metabolism and lipid accumulation due to poor mitophagy, resulting in obesity, hepatic steatosis. Arrowheads denote stimulation whereas the lines with a “T” ending represent inhibition.