Abstract
Background
Inactivated COVID-19 vaccines are safe and effective in the general population with intact immunity. However, their safety and immunogenicity have not been demonstrated in people living with HIV (PLWH).
Methods
42 HIV-1 infected individuals who were stable on combination antiretroviral therapy (cART) and 28 healthy individuals were enrolled in this open-label two-arm non-randomized study at Hubei Provincial Center for Disease Control and Prevention, China. Two doses of an inactivated COVID-19 vaccine (BBIBP-CorV) were given on April 22, 2021 and May 25, 2021, respectively. The reactogenicity of the vaccine were evaluated by observing clinical adverse events and solicited local and systemic reactions. Humoral responses were measured by anti-spike IgG ELISA and surrogate neutralization assays. Cell-mediated immune responses and vaccine induced T cell activation were measured by flow cytometry.
Findings
All the HIV-1 infected participants had a CD4+ T cell count >200 cells/μL both at baseline (659·0 ± 221·9 cells/μL) and 4 weeks after vaccination (476·9 ± 150·8 cells/μL). No solicited adverse reaction was observed among all participants. Similar binding antibody, neutralizing antibody and S protein specific T cell responses were elicited in PLWH and healthy individuals. PLWH with low baseline CD4+/CD8+ T cell ratios (<0·6) generated lower antibody responses after vaccination than PLWH with medium (0·6∼1·0) or high (≥1·0) baseline CD4+/CD8+ T cell ratios (P<0·01). The CD3+, CD4+ and CD8+ T cell counts of PLWH decreased significantly after vaccination (P<0·0001), but it did not lead to any adverse clinical manifestation. Moreover, we found that the general HIV-1 viral load among the PLWH cohort decreased significantly after vaccination (P=0·0192). The alteration of HIV-1 viral load was not significantly associated with the vaccine induced CD4+ T cell activation (P>0·2).
Interpretation
Our data demonstrated that the inactivated SARS-CoV-2 vaccine was safe, immunogenic in PLWH who are stable on cART with suppressed viral load and CD4+ T cell count > 200 cells/μL. However, the persistence of the vaccine-induced immunities in PLWH need to be further investigated.
Research in Context.
Evidence before this study
The safety and efficacy of inactivated COVID-19 vaccines have been validated in general population with intact immunity. We searched PubMed from Nov 1, 2020 to Nov 5, 2021, with the search terms “HIV” AND “Inactivated SARS-CoV-2 vaccine”, no cohort study demonstrating the safety and immunogenicity of inactivated COVID-19 vaccines in people living with HIV (PLWH) had been published.
Added value of this study
Our study provides the first evidence to show humoral and cellular immune responses to an inactivated vaccine in PLWH who have been stable on combination antiretroviral therapy (cART) with good CD4 cell counts. We found that participants with HIV-1 generated antibody and T cell responses comparable with those of healthy individuals after two-dose vaccination. The baseline CD4/CD8 ratios while not the absolute CD4+ T cell counts were shown to be associated with the magnitudes of vaccine induced antibody responses. Moreover, we showed that the vaccine induced T cell activation did not increase the viral burden in PLWH on cART. On the contrary, the levels of plasma HIV-1 RNA decreased among a significant percentage of PLWH.
Implications of all the available evidence
Our data demonstrate that the inactivated COVID-19 vaccine is safe and immunogenic in PLWH who are stable on cART with unsuppressed CD4 counts and indicate that this vaccine might be protective and efficacious against COVID-19 for people with HIV.
Alt-text: Unlabelled box
Introduction
Even though the well-controlled HIV infection per se is not found as a risk factor of increased SARS-CoV-2 prevalence, [1,2] it is alerted that the compromised immunities and the high frequencies of comorbidities may render excessive challenges to this population [3,4] A more recently released WHO report (WHO reference number: WHO/2019-nCoV/Clinical/HIV/2021.1) suggests that HIV infection appears to be a significant independent risk factor for both severe/critical COVID-19 presentation at hospital admission and in-hospital mortality. Vaccination against SARS-CoV-2 for PLWH has also been recommended by WHO and health authorities of many countries. However, as a clinical observation showed that SARS-CoV-2 natural infection induced lower protective antibody responses in people with HIV, [5] it is concerning that the immunogenicity of COVID-19 in PLWH might be weak. The concern escalated when the Novavax COVID-19 vaccine study suggested that HIV infection might dampen the vaccine effectiveness [6].
Reassuringly, more recently published data showed that both the messenger RNA vaccine and the ChAdOx1 nCoV-19 vaccine could elicit protective antibody responses in PLWH comparable with those in healthy individuals [[7], [8], [9], [10]]. To investigate the immunogenicity of inactivated COVID-19 vaccines in PLWH, in this study, we compared the immune responses to two-dose inactivated SARS-CoV-2 vaccination (BIBP-CorV) between healthy vaccinees and HIV-1 infected individuals stable on cART. The safety and effectiveness of BBIBP-CorV have been shown in general population with intact immunity [11], [12], [13], [14]. Here, we further demonstrated that the inactivated COVID-19 vaccine was immunogenic and safe in PLWH. Baseline CD4+/CD8+ T cell ratios were found to be associated with both the RBD binding antibody and the neutralizing antibody responses in PLWH.
Materials and methods
Study design and participants
In this open-label two-arm non-randomized study, we enrolled a cohort of HIV-1 infected individuals (n=42) who were stable on cART and under routine follow-up at Hubei Provincial Center for Disease Control and Prevention, China and a cohort of healthy individuals (n=28). All participants enrolled in this study did not get vaccinated against or infected by SARS-CoV-2 previously, which was manifested by the negative antibody response against SARS-CoV-2 as measured by the Wantai SARS-CoV-2 Ab Rapid Test kit (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., China). Exclusion criteria included pregnancy or lactation, allergy to any vaccine ingredient and comorbidities such as tumor and autoimmune diseases. An additional inclusion criterion for PLWH was CD4+ T cells counts >200 cells/μL. Written informed consent was obtained from all participants, and the study was approved by the Research Ethics Committee of Hubei CDC (approval reference number: HBCDC-AF/SC-08/02.0).
BBIBP-CorV was developed by the Beijing Institute of Biological Products (Beijing, China) [15]. Two doses of the vaccine (4 μg/dose) were administered via deltoid intramuscular injection on April 22, 2021 and May 25, 2021, respectively.
The overview of the study protocol was shown in Supplementary figure 1.
Peripheral lymphocyte count and plasma HIV-1 viral RNA detection
The peripheral lymphocyte counts and plasma HIV-1 viral RNA detections for HIV-1 infected individuals were performed using the BD Multitest™ CD3 FITC / CD8 PE / CD45 PerCP / CD4 APC reagent and the COBAS® AmpliPrep / COBAS® TaqMan® HIV-1 Test (v2·0) kit, respectively. Samples collected both at the baseline and 4 weeks after the 2nd dose of vaccination were measured following the manufacturers` instructions in the clinical laboratory of Shanghai Public Health Clinical Center. The COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test (v2·0) kit has a linear range of 20–1 × 107 HIV-1 RNA copies/mL. Samples with detected Ct values below the lowest limit of the linear range were defined as HIV-1 viral RNA load <20 copies/mL, while samples without detected Ct values were defined as HIV-1 viral RNA load undetectable.
Titration of SARS-CoV-2 RBD binding antibody
SARS-CoV-2 RBD specific binding antibodies were detected by an in-house enzyme-linked immunosorbent assay (ELISA) at the baseline, after the 1st dose and after the 2nd dose, respectively. High-binding 96-well EIA plates (Cat# 9018, Corning, USA) were coated with purified SARS-CoV-2 RBD protein (Cat# 40591-V08H, Sino Biological, China) at a final concentration of 1µg/ml in carbonate/bi-carbonate coating buffer (30mM NaHCO3,10mM Na2CO3, pH 9·6). Subsequently, the plates were blocked with 1 × PBS containing 5% skimmed milk for 1 hour at 37°C. Next, 50μl of human plasma diluted with dilution buffer (1 × PBS containing 5% skimmed milk) was added to each well. After 1-hour incubation at 37°C, the plates were washed with 1 × PBS containing 0·05% Tween20 for 5 times. Then, 50μl of an HRP labeled goat anti-human IgG antibody (Cat# ab6759, Abcam, UK) diluted in 1 × PBS containing 5% skimmed milk were added to each well and incubated for 1 hour at 37°C. After a second round of wash, 50μl of TMB substrate reagent (Cat# MG882, MESGEN, China) was added to each well. 15 minutes later, the color development was stopped by adding 50μl of 1M H2SO4 to each well and the values of optical density at OD450nm and OD630nm were measured using 800 TS microplate reader (Cat# 800TS, Biotek, USA). The cut-off value was defined as 3-fold of the average OD450-630 of background control (1 × PBS containing 5% skimmed milk) wells. Titers expressed the degree to which the plasma can be diluted, and the OD450-630 value still exceed the cut-off value.
Quantification of SARS-CoV-2 neutralizing antibodies
A commercialized surrogate neutralization test developed by Suzhou Xinbo Biotechnology Ltd. Company (PerkinElmer, China) was employed to measure neutralizing antibodies against SARS-CoV-2 at the baseline, after the 1st dose and after the 2nd dose, respectively. The rationale of this assay is based on antibody-mediated blockage of the interaction between the angiotensin-converting enzyme 2 (ACE2) receptor protein and the receptor-binding domain [16]. Its performance and reliability have been verified by multiple previous studies [17], [18], [19]. Briefly, plasma samples were firstly incubated with acridinium ester labeled SARS-CoV-2 RBD for 15 min at room temperature. Then, magnetic beads coated with purified human ACE2 protein were added into the mixture and incubated for 15 min. After washing, the acridinium ester labeled RBD bound with the magnetic beads were measured by a chemiluminescent reaction. The concentrations of SARS-CoV-2 neutralizing antibody correlate negatively with the luminescence intensities, which can be quantified by establishing a standard curve.
Flowcytometry assays
To minimize measurement errors, freshly isolated PBMCs were preserved in liquid nitrogen until the last follow-up was completed. Upon detection, the PBMCs isolated at the baseline and 4 weeks post the 2nd dose were recovered and washed twice with R10 (RPMI1640 with 10% FBS). After counting, 1 million live (trypan blue negative) cells from each sample were added to 96-well round bottom plates and incubated with either R10 or R10 containing synthesized spike protein peptides (0.6μg/ml for each peptide) overnight. Then, the cells were washed and stained sequentially with live/dead dye(Zombie Aqua Fixable Viability Kit, cat#423101, BioLegend), surface marker antibodies (Anti-human-CD3-APC/cyanine 7, cat#300318, BioLegend; anti-human-CD4-BV605, cat#566148, BioLegend; anti-human-CD8-APC, cat#344722, BioLegend; anti-human-HLA-DR-BV421, cat#307636, BioLegend; anti-human-CD38-Percp-Cy5·5, cat#551400, BD Pharmingen) and an intracellular IFN-γ antibody (anti-human-IFN-γ-PE, cat#502509, BioLegend). Stained samples were analyzed using a BD Fortessa flow cytometer and data were analyzed with FlowJo software version 10 (TreeStar Inc., Ashland, OR, USA). The gating strategy is shown in Supplementary figure 2. The evaluation of T cell activation was based on the data generated from wells incubated with R10.
Statistical analysis
Quantitative values were examined for normality using the Shapiro–Wilk test before all downstream analyses except the Chi square test. Means for variables with a normal distribution were compared using the two-tailed parametric t test and with the two-tailed non-parametric t-test when distributions of data departed from normality. In before-after analyses, paired data were interrogated using the Wilcoxon signed-rank test for nonparametric data and paired t test for parametric data. Differences among multiple groups were compared by the method of one-way ANOVA. The contingency analysis was done using the method of Chi-square test. Participants who are loss to follow up are excluded from statistical analysis. All the statistical analyses were conducted using Graphpad Prism 9 (GraphPad Software, USA).
Role of the funding source
The funding source was not involved in study design, data collection and analysis, data interpretation, the writing of the report, and the decision to submit the paper for publication. All authors had full access to all the data. The decision of submission was made by the corresponding authors (YMW and HT).
Results
Baseline characteristics of PLWH and HIV related events after vaccination
In this study, 28 healthy individuals and 42 PLWH were inoculated with two doses of the inactivated vaccine (BIBP-CorV) produced by Beijing Institute of Biological Products on 22 April 2021 and 25 May 2021, respectively. PLWH were slightly older than the control (42·74 ± 10·17 versus 37·79 ± 8·804 years, P=0·0392). The gender composition (29 Man, 13 Woman versus 16 Man, 12 Woman; P=0·3228) and the mean BMI (23·97 ± 6·967 versus 24·07 ± 3·8, P=0·2867) were similar between PLWH and healthy individuals (Table 1). All the HIV-1 infected individuals have been on cART since being diagnosed with HIV-1 and have a peripheral CD4+ T cell count of >200 cells/μL. No solicited adverse reaction was observed among all participants. None of PLWH developed HIV-related clinical events during the study period from 22 April 2021 to 25 June 2021.
Table 1.
Demographical characteristics of enrolled vaccinees
| PLWH | HC | P value | |
|---|---|---|---|
| Gender | |||
| Man (n) | 29 | 16 | 0·3228 |
| Woman (n) | 13 | 12 | |
| Age (Year, mean±SD) | 42·74±10·17 | 37·79±8·804 | 0·0392 |
| BMI (Mean±SD) | 23·97±6·967 | 24·07±3·8 | 0·2867 |
Note: PLWH, people living with HIV; HC, healthy control
At the beginning of vaccination, 22 patients had an undetectable plasma viral load, 8 patients had detectable viral loads below 20 copies/ml and 12 patients had detectable viral loads beyond 20 copies/ml (21-34,700 copies/mL) (Table 2). Unexpectedly, we found that the general HIV-1 viral load among PLWH decreased after vaccination: 34 patients had an undetectable plasma viral load, 4 patients had detectable viral loads below 20 copies/ml and 4 patients had detectable viral loads beyond 20 copies/ml (24·4–161,000 copies/mL) (P=0·0192) (Table 2). Meanwhile, our data also showed that the peripheral T cell counts decreased significantly after vaccination, but the CD4+/CD8+ T cell ratios remained stable (Table 2).
Table 2.
Peripheral T cell counts and plasma viral loads of PLWH
| Baseline | 4 weeks post the 2nd dose | P value | |
|---|---|---|---|
| T cell count (Mean ± SD cells/μL, n) | |||
| CD3+ T | 1550 ± 502, 28 | 1121 ± 336, 28 | Paired t-test, <0·0001 |
| CD4+ T | 659 ± 222, 28 | 477 ± 151, 28 | Paired t-test, <0·0001 |
| CD8+ T | 813 ± 361, 28 | 586 ± 240, 28 | Paired t-test, <0·0001 |
|
CD4+ T/CD8+ T ratio (Median, 25%-75% percentile, n) |
0·86, 0·60-1·05, 28 | 0·82, 0·62-1·25, 28 | Paired rank test, 0·5194 |
| Plasma viral load | |||
| >20 copies/mL (n) | 12 | 4 | Chi square test, 0·0192 |
| <20 copies/mL (n) | 8 | 4 | |
| Undetectable (n) | 22 | 34 |
The inactivated SARS-CoV-2 vaccine elicited comparable antibody and T cell responses in PLWH with those in healthy individuals
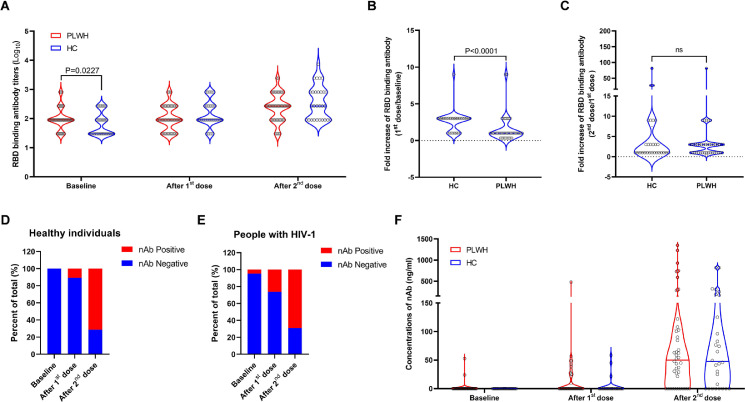
To evaluate the immunogenicity of the inactivated SARS-CoV-2 vaccine in our cohorts, peripheral blood samples were collected at baseline, 4 weeks after the first dose and 4 weeks after the second dose, respectively. The results of RBD binding antibody assays showed that the inactivated vaccine induced similar levels of RBD binding antibodies in the two cohorts after both the first and the second dose (Fig.1A). We found that the average fold increase of RBD binding antibody was significantly lower in PLWH (Fig.1B) and the responding rates (defined as ≥3-fold increase from the baseline) were also significantly lower in PLWH after both the first (P<0·0001) and the second (P=0·0001) dose (Supplementary table 1), which was presumably due to the relatively higher background antibody levels in this cohort (Fig.1A). Given that all participants were not infected by or vaccinated against SARS-CoV-2 previously, we speculated that the baseline RBD binding antibodies were probably induced by previous exposures to seasonal human coronaviruses. Neutralizing antibody responses were elicited in most participants after two doses of vaccination (Fig. 1D and 1E). Both the magnitudes (Post the 1st dose, P=0·1021; post the 2nd dose, P=0·9640) (Fig. 1F) and the responding rates (defined as ≥3-fold increase from the baseline) (Post the 1st dose, P=0·2183; post the 2nd dose, P>0·9999) (Supplementary table 2) were similar between PLWH and the healthy control. In addition, we also found that the mean levels of S2 binding antibodies were similar between PLWH and healthy individuals both at baseline (P=0·5062) and 4 weeks post second vaccination (P=0·0877) (Supplementary Figure 3).
Figure 1.
The inactivated SARS-CoV-2 vaccine elicited comparable RBD binding antibody and neutralizing antibody responses in PLWH with those in the control. All participants in our study received two doses of the inactivated SARS-CoV-2 vaccine (BIBP-CorV) produced by Beijing Institute of Biological Products at an interval of 33 days. Peripheral blood samples were collected at baseline, 4 weeks post the 1st dose and 4 weeks post the 2nd dose. RBD binding antibody (A, B, C) and neutralizing antibody (D, E, F) responses were measured and compared between PLWH (n=28) and healthy individuals (n=42). Statistical analyses were performed by the method of non-parametric t test.
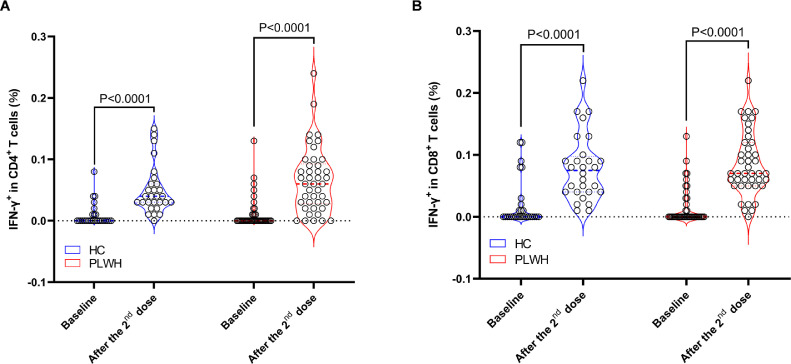
The results of flowcytometry assays showed that two-dose inactivated SARS-CoV-2 vaccine induced significantly higher T cell responses in both HC (P<0·0001) and PLWH (P<0·0001) compared to the baseline (Fig.2). Similar magnitudes of S protein specific CD4+ (0·04%, IQR 0·03%-0·06% vs 0·062%, IQR 0·028%-0·093%, P=0·2024) (Fig. 2A) and CD8+ (0·08%, IQR 0·04%-0·10% vs 0·09%±0·05%, P=0·4750) (Fig. 2B) T cell responses were observed in the two cohorts.
Figure 2.
Comparisons of S protein specific T cell responses between PLWH and healthy individuals. S protein specific IFN-γ secreting T cells were measured using flowcytometry. The inactivated vaccine elicited similar levels of S protein specific CD4+ (A) and CD8+ T cell responses (B) in PLWH and HC. Statistical analyses were performed by the method of non-parametric t test.
We noticed the difference in age between PLWH and HC. To clarify the potential impact of the age difference on immune responses, the HC and PLWH groups were further divided into two subgroups (<40 y and ≥40 y), respectively. And the results of comparisons showed that, in both age groups, the vaccine induced antibody and T cell responses were similar between PLWH and HC (Supplementary figure 4).
Baseline CD4/CD8 ratios were associated with antibody responses after the vaccination in the cohort of PLWH
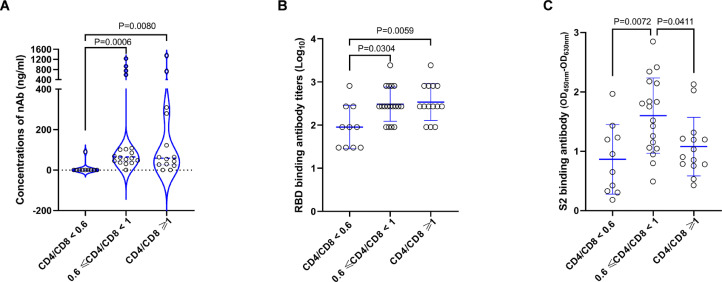
To understand whether the baseline immune status can influence the vaccine induced antibody responses in PLWH, we further analyzed the relationships between baseline T cell counts and post vaccination antibody responses. Our data showed that neither the CD4+ T cell counts nor the CD8+ T cell counts at baseline correlated with the vaccine induced antibody responses (Supplementary figure 5). While, the CD4+/CD8+ T cell ratios at baseline were found to be associated with antibody responses after the second dose of vaccination (Fig.3). More specifically, the average RBD binding and neutralizing antibody responses of HIV-1 positive vaccinees with low baseline CD4+/CD8+ T cell ratios (<0·6) were significantly lower than those with medium (0·6-1·0) or high (≥1·0) baseline CD4+/CD8+ T cell ratios (Fig.3A and 3B). Slightly different from the RBD binding antibody and the nAb responses, we observed that S2 binding antibody responses in HIV-1 positive vaccinees with medium baseline CD4+/CD8+ T cell ratios were significantly higher than those with both low and high baseline CD4+/CD8+ T cell ratios (Fig.3C). We also analyzed the impacts of baseline CD4+/CD8+ T cell ratios on T cell responses and found that there was no significant difference among PLWH with low, medium and high baseline CD4+/CD8+ T cell ratios (Supplementary figure 6).
Figure 3.
Baseline CD4+/CD8+ T cell ratios were associated with specific antibody responses after vaccination in PLWH. HIV-1 infected participants were stratified into 3 subgroups according to their baseline CD4+/CD8+ T cell ratios (<0•6, 0•6∼1•0 and ≥1•0). RBD binding antibody (A), neutralizing antibody (B) and S2 binding antibody (C) responses were compared among the three subgroups. Statistical analysis for (A) was performed by the method of non-parametric t test and statistical analyses for (B) and (C) were done by the method of parametric t test. Median ± IQR of each group is shown in (A). Mean ± SD of each group is shown in (B) and (C).
The patterns of vaccine induced T cell activation were different between HC and PLWH
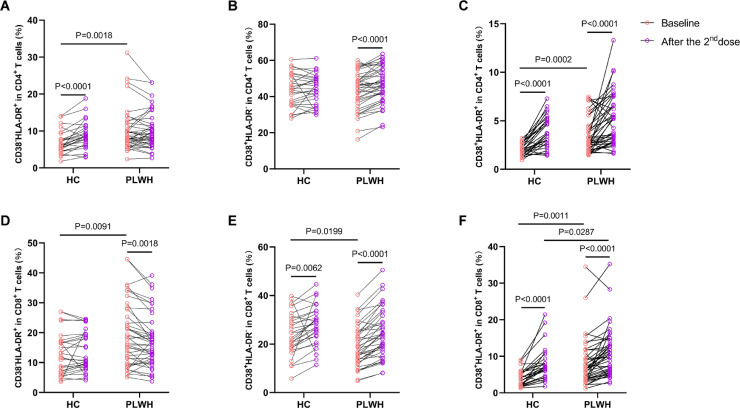
Vaccine induced activation of T cells was considered as a major constraint that might compromise vaccine conferred benefits in PLWH.[20], [21], [22] To investigate whether the vaccine induced T cell activation was associated with the observed fluctuation of HIV-1 viral load, we measured the expressions of CD38 and HLA-DR on CD4+ and CD8+ T cells by flowcytometry. We observed that the patterns of T cell activation were different between HC and PLWH (Fig.4). Of note, our data showed that the percentages of CD38+HLA-DR− CD4+ T cells (Fig.4B) increased, while the percentages of CD38−HLA-DR+ CD8+ T cells (Fig.4D) decreased in PLWH after vaccination. The decrease of CD38−HLA-DR+CD8+ T cells has been reported by a previous study,20 however, the underlying mechanism is not clear. Additionally, our data also showed that the baseline frequencies of CD38−HLA-DR+CD4+ T cells (P=0·0018), CD38+HLA-DR+CD4+ T cells (P=0·0002), CD38−HLA-DR+CD8+ T cells (P=0·0091), CD38+HLA-DR−CD8+ T cells (P=0·0199) and CD38+HLA-DR+CD8+ T cells (P=0·0011) were significantly higher in the PLWH cohort. After two-dose vaccination, the frequency of CD38+HLA-DR+CD8+ T cells was significantly higher in the PLWH cohort. We further analyzed the association between the observed T cell activation and plasma viral load fluctuation. Our results showed that the vaccine induced fold-changes of CD38+HLA-DR− (P=0·2277), CD38−HLA-DR+ (P=0·5464) and CD38+HLA-DR+CD4+ (P=0·3045) T cells were not found to be significantly higher in viral increased PLWH (Supplementary figure 7). Of note, no statistically significant linear correlation was observed between CD4+/CD8+ T cell ratios and vaccine induced immune responses (Supplementary figure 8).
Figure 4.
The patterns of vaccine induced T cell activation were different between PLWH and HC. The activation of T cells was assessed through detecting the expressions of CD38 and HLA-DR on T cells. (A, B, C) The percentages of CD38−HLA-DR+, CD38+HLA-DR− and CD38+HLA-DR+ among CD4+ T cells. (D, E, F) The percentages of CD38−HLA-DR+, CD38+HLA-DR− and CD38+HLA-DR+ among CD8+ T cells. Statistical analyses were performed by the method of two-way ANOVA.
Discussion
Even if the replication of HIV is controlled by cART, it can still be harder for PLWH to defend against infectious diseases [23]. Therefore, vaccination is recommended for PLWH whose peripheral CD4+ T cell counts are more than 200 cells/μL [24]. However, due to the compromised immune status, the vaccine elicited immune responses might be impaired in PLWH [[25], [26], [27], [28]]. The efficacies of COVID-19 vaccines in PLWH were also seriously concerned, because it has been suggested that HIV-1 infection could alter the host immune responses against SARS-CoV-2 [29]. Here, we provided evidence showing that an inactivated SARS-CoV-2 vaccine (BIBP-CorV) was immunogenic and safe in PLWH. Importantly, our data demonstrated that the inactivated vaccine induced comparable RBD binding antibody, neutralizing antibody and S protein specific T cell responses between PLWH and HC.
All the HIV-1 positive participants in our cohort had a CD4+ T cell count of >200 cells/μL both at baseline and 4 weeks post vaccination. We found a statistically significant decrease in CD4+ T cell count between the baseline levels and those measured following the second vaccination. The decrease of CD4+ T cell count after vaccination had also been observed in PLWH inoculated with the BNT162b2 mRNA vaccine and several other vaccines usually recommended for PLWH [10,30]. Increases in activated T cells were found to be associated with decreases in CD4+ T cell count among PLWH received vaccination, [30] but the reason is still not clear. Of note, both our and other studies did not identify any adverse clinical manifestation that was associated with the drop of CD4+ T cell count [30]. Stable cART might be crucial to counteract the potential vaccine related deleterious effect in PLWH according to a recently published case report, which observed viral activation and CD4+ T cell loss in a treatment-naïve HIV-positive patient after receiving two doses of inactivated COVID-19 vaccines [31].
Quite surprisingly, we observed a significant drop of general HIV-1 viral load in the PLWH cohort after vaccination. As percentages of activated T cells may correlate with HIV-1 replication, we further evaluated the vaccine induced T cell activation by measuring the expressions of CD38 and HLA-DR on T cells. We found that the percentages of CD38+HLA-DR−CD4+ T cells and CD38+HLA-DR+CD4+ T cells increased significantly after vaccination. We speculate that the elimination of activated HIV-1 infected CD4+ T cells by cART or autologous CTLs might account for the observed drop of HIV viral load in the PLWH cohort.
Despite the generally comparable antibody responses between PLWH and healthy individuals, we demonstrated that HIV-1 infected vaccinees with low baseline CD4+/CD8+ T cell ratio (<0·6) generated weaker antibody responses after vaccination, which was in line with previous reports showing that CD4+/CD8+ T cell ratios could predict vaccine induced antibody responses in HIV-1 infected patients and HIV negative elderly [32,33]. Additionally, we showed that the baseline CD4+/CD8+ T cell ratios did not impact the induction of specific T cell responses. Given that most vaccine induced antibody responses are CD4+ T cell dependent, a potential explanation for the above phenomena is that the low CD4+/CD8+ T cell ratio might reflect the suboptimal support for antibody responses.
Previous cohort studies suggested that a CD4/CD8 ratio <0·3 was associated with an increased risk of non-AIDS-related events among HIV infected patients compared with one > 0·45 [34,35]. In this study, we were not able to do the analysis by stratifying the PLWH cohort into subgroups with a CD4/CD8 ratio <0·3, 0·3 -0·45, or >0·45, because most patients (38 out of 42, 90·5%) in this study had a CD4/CD8 ratio >0·45. Nonetheless, our conclusion is generally in consistent with previous studies.
Several limitations should be noted. First, the inadequate follow-up does not allow us to observe the persistence of specific immune responses and to assess potential long-term adverse effects caused by decreased T cell counts. Second, the limited sample size does not support a reliable safety evaluation and make it hard to do extensive subanalyses. Third, our study is confined to PLWH with a CD4+ T cell count >200 cells/μL, therefore the vaccine immunogenicity among PLWH with unsuppressed viremia or advanced immunosuppression needs to be further investigated. Fourth, the participants enrolled in this study are under 60 years old, therefore the vaccine immunogenicity in PLWH older than 60 years also need to be further clarified. Fifth, due to the lack of a non-vaccinated PLWH group, the mechanism underlying the decrease of HIV-1 viral load after vaccination is still unclear. Besides, the neutralizing activities against emerging SARS-CoV-2 variants, such as the delta lineage, were not evaluated in this study. Despite of these limitations, our study demonstrates that the inactivated COVID-19 vaccine was immunogenic and safe in PLWH who are under 60 years old and stable on cART.
Author contributions
YW and HT were involved in the study design and supervision; YF, YZ, ZH, XT, GW, DC and LJ were involved in data collection; YR, WW, JW, LS, WZ and YW performed the data analysis; YW, YF and HT drafted the manuscript; WZ, LS, YW and HT revised the manuscript. HH coordinated the enrollment of PLWH. The underlying data have been verified by YF and YW.
Data sharing statement
Anonymized participant-level data will be made available 3 months following publication, upon requests directed to the corresponding author. After being approved by the sponsors (Fudan University and Hubei provincial CDC) and investigators, the data can be shared through a secure online platform. All data will be made available for a minimum of 5 years after publication.
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 81971559, 82041010), Fudan University and Hubei provincial CDC.
Declaration of Competing Interest
The authors have nothing to declare.
Acknowledgments
We thank Dr. Lianguo Ruan from Wuhan Jinyintan Hospital for his help in PLWH cohort enrollment. We thank the volunteers who participated in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101226.
Contributor Information
Heng Tang, Email: 867555881@qq.com.
Yanmin Wan, Email: yanmin_wan@fudan.edu.cn.
Appendix. Supplementary materials
References
- 1.Kanwugu ON, Adadi P. HIV/SARS-CoV-2 coinfection: A global perspective. Journal of medical virology. 2021;93(2):726–732. doi: 10.1002/jmv.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosioni J, Blanco JL, Reyes-Urueña JM, et al. Overview of SARS-CoV-2 infection in adults living with HIV. The lancet HIV 2021; 8(5): e294-e305. [DOI] [PMC free article] [PubMed]
- 3.Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID-19 Among People Living with HIV: A Systematic Review. AIDS and Behavior. 2021;25(1):85–92. doi: 10.1007/s10461-020-02983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper TJ, Woodward BL, Alom S, Harky A. Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: a systematic review. HIV medicine. 2020;21(9):567–577. doi: 10.1111/hiv.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinelli MA, Lynch KL, Yun C, et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study. The lancet HIV 2021; 8(6): e334-e41. [DOI] [PMC free article] [PubMed]
- 6.Callaway E, Mallapaty S. Novavax offers first evidence that COVID vaccines protect people against variants. Nature. 2021;590(7844):17. doi: 10.1038/d41586-021-00268-9. [DOI] [PubMed] [Google Scholar]
- 7.Woldemeskel BA, Karaba AH, Garliss CC, et al. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with HIV. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2021:ciab648. doi: 10.1093/cid/ciab648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruddy JA, Boyarsky BJ, Bailey JR, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS (London, England) 2021;35(14):2399–2401. doi: 10.1097/QAD.0000000000003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. The lancet HIV 2021; 8(8): e474-e85. [DOI] [PMC free article] [PubMed]
- 10.Levy I, Wieder-Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2021 doi: 10.1016/j.cmi.2021.07.031. S1198-743X(21)00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. The Lancet Infectious Diseases. 2021 doi: 10.1016/S1473-3099(21)00462-X. S1473-3099(21)00462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeewandara C, Aberathna IS, Pushpakumara PD, et al. Persistence of antibody and T cell responses to the Sinopharm/BBIBP-CorV vaccine in Sri Lankan individuals. medRxiv: the preprint server for health sciences 2021. 2021 10.14.21265030. [Google Scholar]
- 14.Xia S, Duan K, Zhang Y, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. Jama. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Zhang Y, Huang B, et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020;182(3):713–721. doi: 10.1016/j.cell.2020.06.008. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nature Biotechnology. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 17.Chan K-H, Leung K-Y, Zhang R-R, et al. Performance of a Surrogate SARS-CoV-2-Neutralizing Antibody Assay in Natural Infection and Vaccination Samples. 2021;11(10):1757. doi: 10.3390/diagnostics11101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Rhein C, Scholz T, Henss L, et al. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. Journal of virological methods. 2021;288 doi: 10.1016/j.jviromet.2020.114031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohmer N, Rühl C, Ciesek S, Rabenau HF. Utility of Different Surrogate Enzyme-Linked Immunosorbent Assays (sELISAs) for Detection of SARS-CoV-2 Neutralizing Antibodies. Journal of clinical medicine. 2021;10(10) doi: 10.3390/jcm10102128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onlamoon N, Unpol P, Boonchan M, et al. Immune activation and viral replication after vaccination with an influenza A H1N1 2009 vaccine in HIV-infected children receiving antiretroviral therapy. Disease markers. 2013;35(4):221–227. doi: 10.1155/2013/276547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Eller MA, Zafar S, et al. Preferential infection of human Ad5-specific CD4 T cells by HIV in Ad5 naturally exposed and recombinant Ad5-HIV vaccinated individuals. 2014;111(37):13439–13444. doi: 10.1073/pnas.1400446111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Immunization with Both T Cell-Dependent and T Cell-Independent Vaccines Augments HIV Viral Load Secondarily to Stimulation of Tumor Necrosis Factor α. 1998; 14(9): 727-34. [DOI] [PubMed]
- 23.Crum-Cianflone NF, Wallace MR. Vaccination in HIV-infected adults. AIDS patient care and STDs. 2014;28(8):397–410. doi: 10.1089/apc.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frésard A, Gagneux-Brunon A, Lucht F, Botelho-Nevers E, Launay O. Immunization of HIV-infected adult patients - French recommendations. Human vaccines & immunotherapeutics. 2016;12(11):2729–2741. doi: 10.1080/21645515.2016.1207013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck CR, McKenzie BC, Hashim AB, et al. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis from a public health policy perspective. PloS one. 2011;6(12):e29249. doi: 10.1371/journal.pone.0029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tebas P, Frank I, Lewis M, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS (London, England) 2010;24(14):2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 27.George VK, Pallikkuth S, Parmigiani A, et al. HIV infection Worsens Age-Associated Defects in Antibody Responses to Influenza Vaccine. The Journal of infectious diseases. 2015;211(12):1959–1968. doi: 10.1093/infdis/jiu840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansoor N, Scriba TJ, de Kock M, et al. HIV-1 Infection in Infants Severely Impairs the Immune Response Induced by Bacille Calmette-Guérin Vaccine. The Journal of infectious diseases. 2009;199(7):982–990. doi: 10.1086/597304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karim F, Gazy I, Cele S, et al. HIV infection alters SARS-CoV-2 responsive immune parameters but not clinical outcomes in COVID-19 disease. medRxiv. 2020 2020.11.23.20236828. [Google Scholar]
- 30.Castro P, Plana M, Gonzalez R, et al. Influence of a vaccination schedule on viral load rebound and immune responses in successfully treated HIV-infected patients. AIDS Res Hum Retroviruses. 2009;25(12):1249–1259. doi: 10.1089/aid.2009.0015. [DOI] [PubMed] [Google Scholar]
- 31.Gong C, Song X, Li X, Lu L, Li T. Immunological Changes after COVID-19 Vaccination in an HIV-Positive Patient. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2021 doi: 10.1016/j.ijid.2021.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avelino-Silva VI, Miyaji KT, Hunt PW, et al. CD4/CD8 Ratio and KT Ratio Predict Yellow Fever Vaccine Immunogenicity in HIV-Infected Patients. PLoS neglected tropical diseases. 2016;10(12) doi: 10.1371/journal.pntd.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strindhall J, Ernerudh J, Mörner A, et al. Humoral response to influenza vaccination in relation to pre-vaccination antibody titres, vaccination history, cytomegalovirus serostatus and CD4/CD8 ratio. Infectious diseases (London, England) 2016;48(6):436–442. doi: 10.3109/23744235.2015.1135252. [DOI] [PubMed] [Google Scholar]
- 34.Mussini C, Lorenzini P, Cozzi-Lepri A, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. The lancet HIV. 2015;2(3):e98–106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 35.Wolday D, Kebede Y, Legesse D, et al. Role of CD4/CD8 ratio on the incidence of tuberculosis in HIV-infected patients on antiretroviral therapy followed up for more than a decade. PloS one. 2020;15(5) doi: 10.1371/journal.pone.0233049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.