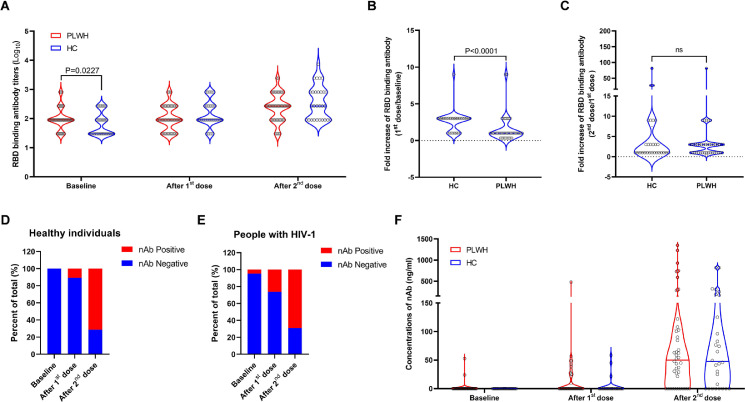
Figure 1.
The inactivated SARS-CoV-2 vaccine elicited comparable RBD binding antibody and neutralizing antibody responses in PLWH with those in the control. All participants in our study received two doses of the inactivated SARS-CoV-2 vaccine (BIBP-CorV) produced by Beijing Institute of Biological Products at an interval of 33 days. Peripheral blood samples were collected at baseline, 4 weeks post the 1st dose and 4 weeks post the 2nd dose. RBD binding antibody (A, B, C) and neutralizing antibody (D, E, F) responses were measured and compared between PLWH (n=28) and healthy individuals (n=42). Statistical analyses were performed by the method of non-parametric t test.