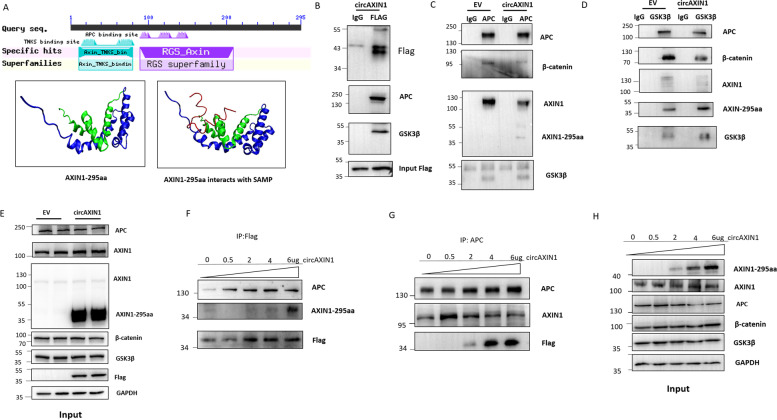
Fig. 7.
AXIN1-295aa competitively binds to APC. a Upper panel: Sequence alignment reveals that AXIN1-295aa contains an RGS domain. Lower panels: The 3D structure shows the simulated binding interface between AXIN1-295aa and the SAMP structure of APC, created using the online software packages PHYRE and ZDOCK. The left image shows the predicted 3D structure of AXIN1-295aa. The red line is the SAMP domain of APC, which is the domain that interacts with full length AXIN1. The right image illustrates the potential interaction interface between the SAMP domain of APC and AXIN1-295aa. b-d A co-IP assay was performed to evaluate the binding potential among AXIN1-295aa, AXIN1, APC, β-catenin, and GSK3β. b AXIN1-295aa binds to APC and GSK3β. c Overexpression of circAXIN1 attenuates the binding between APC with AXIN1 and β-catenin. d Overexpression of circAXIN1 attenuates the binding between GSK3β and β-catenin. e The expression of APC, AXIN1, β-catenin, and GSK3β is unchanged after transfection of circAXIN1 in 293 T cells. f-g AXIN1-295aa competitively binds to APC. f Various doses of circAXIN1 were transfected into 293 T cells. IP using FLAG antibody was performed to detect the change in interaction between APC and AXIN1-295aa. g IP was performed using anti-APC antibody. Binding of APC to AXIN1 is weakened and binding of APC to AXIN1-295aa is enhanced with higher doses of circAXIN1 transfection. h The expression of AXIN1-295aa increased in a dose-dependent manner. The expression of AXIN1, APC, GSK3β, and β-catenin barely changed with the various doses of circAXIN1 transfection