Figure 1.
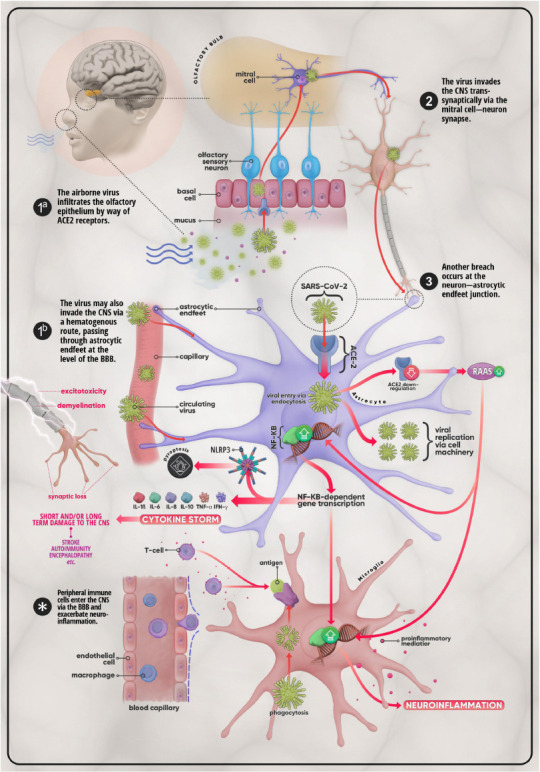
Mechanisms of invasion and possible CNS effects of SARS-CoV-2.
(1a) The virus first enters the body via the nasal passage and makes its way to the olfactory bulb by crossing the olfactory epithelium using ACE-2 receptors. From there the virus enters the CNS through (2) the mitral cell-neuron synapse. (3) The virus then infects astrocytes, ACE-2-expressing glial cells which can also be infected via (1b) astrocytic end-feet that surround endothelial cells at the level of the BBB. In addition to utilizing cell machinery to proliferate, SARS-CoV-2 provokes the downregulation of ACE-2 in host cells, which in turn leads to the over-activation of the RAAS system. This promotes the upregulation of the NF-κB pathway and the subsequent transcription of genes coding for pro-inflammatory mediators such as IL-1β, IL-6, IL-8, IL-10, TNF-α, and IFN-γ. This medley of cytokines, chemokines, and other pro-inflammatory molecules may develop into what is termed the cytokine storm, a life-threatening condition that has the potential to cause short- as well as long-term damage on the CNS in the form of stroke, auto-immune disease, or encephalopathy, etc. Disruption of the BBB and the ensuing infiltration of the CNS by peripheral immune cells (T-cells, macrophages, etc.), triggered by microglial antigen presentation, further excarbates the situation. The overall result is further aggravation of neuroinflammation and injury cascades that include, but are certainly not limited to, synaptic loss, demyelination, and excitotoxicity. ACE-2: Angiotensin-converting enzyme 2; BBB: blood-brain barrier; CNS: central nervous system; IFN: interferon; IL: interleukin; NF-κB: nuclear factor κB; RAAS: renin-angiotensin-aldosterone system; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; TNF-α: tumor necrosis factor-α.
