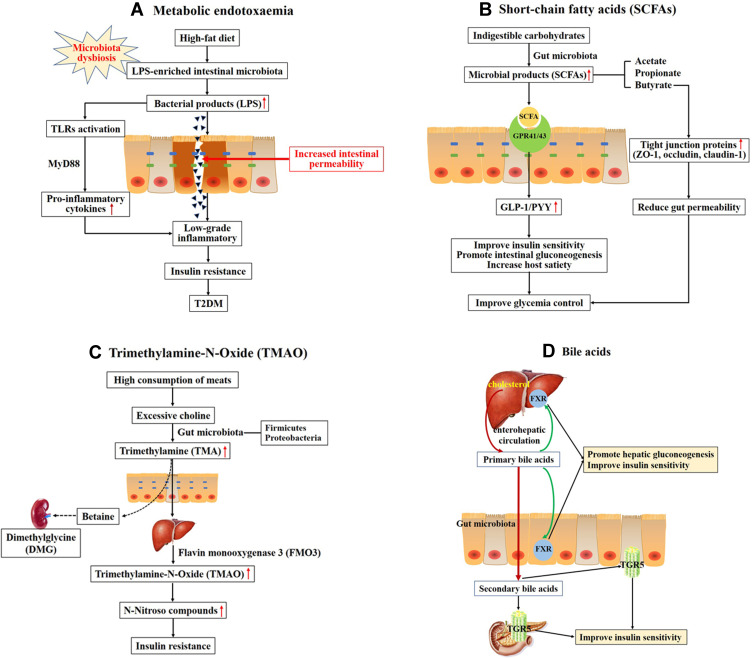
Figure 1.
Overall scheme showing the potential mechanisms linking gut microbiota and the development of T2DM. (A) High-fat diet increases LPS-enriched intestinal microbiota, resulting in elevating the concentration of LPS. Excessive LPS triggers the dysfunction of intestinal barrier and increases intestinal permeability, then leading to the low-grade inflammatory through binding and activating Toll-like receptors (TLRs). Chronic low-grade inflammation is associated with insulin resistance and type 2 diabetes. (B) Indigestible carbohydrates were hydrolyzed and fermented to produce short-chain fatty acids (SCFAs), such as acetate, propionate and butyrate. SCFAs activate G-protein coupled receptors 41 and 43 (GPR41/43) to stimulate production of glucagon-like peptide-1 (GLP-1) and the intestinal peptide YY (PYY), which improve insulin secretion and promote intestinal gluconeogenesis. (C) High consumption of meats leads to produce excessive choline. Choline is metabolized to produce trimethylamine (TMA) by gut microbiota, primarily the Firmicutes and Proteobacteria phyla. TMA readily passes the intestinal wall to form trimethylamine-N-Oxide (TMAO). TMAO promotes insulin resistance through forming N-Nitroso compounds. Choline may escape microbial degradation and converted into betaine and further metabolites (eg, dimethylglycine (DMG)) which have detrimental osmotic effects by mammalian mitochondrial pathways in kidney. (D) Primary bile acids are synthesized from cholesterol in hepatocytes. Secondary bile acids are derived from primary bile acids, mainly by the biosynthetic capabilities of few gut microbes. The primary BAs can regulate hepatic glucose metabolism and insulin sensitivity through activating the nuclear farnesoid X receptors (FXR) in liver and intestine. Secondary bile acids can stimulate GLP-1 secretion from L-cells in the intestine to improve insulin sensitivity through binding to G-protein-coupled bile acid receptor 1 (TGR5) in enteroendocrine cells and pancreatic β-cells.