Fig. 2.
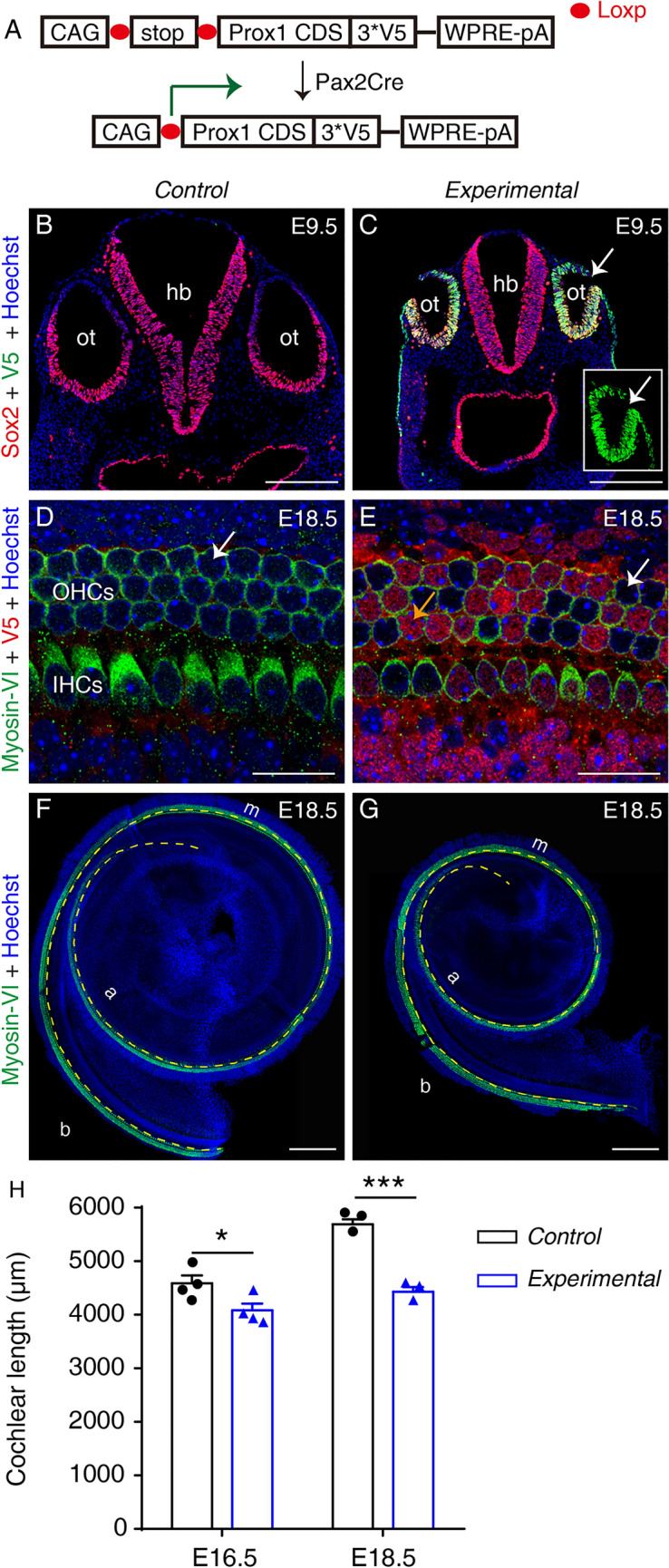
Ectopic Prox1 expression causes cochlear shortening without disrupting HC differentiation by E18.5. A Design of conditional Prox1-overexpression mouse model. Pax2Cre turns on ectopic Prox1 in mouse otocyst cells. B, C Double staining of Sox2 and V5 (Prox1) in the otocyst (ot) in a control Rosa26-LSL-Prox1/+ mouse (B) and an experimental Pax2Cre+; Rosa26-LSL-Prox1/+ mouse (C). Ectopic V5-tagged Prox1 is detectable in experimental but not control otocyst cells (Sox2+). Inset in (C) is the same otocyst (arrow in C) but visualized in V5 panel only. D, E Double staining of Myosin-VI and V5 (Prox1) in whole-mount cochlear samples of a control (D) and an experimental (E) mouse. At E18.5, no HCs in cochlear samples from control mice express V5-tagged Prox1 (arrow in D), whereas in the cochlear sample from an experimental mouse, numerous HCs and other cells (descendants of Pax2+ progenitors) are V5+ (orange and white arrows in E indicate V5+ and V5– HCs). The weak red signal in D is background and the control sample is negative for V5. F, G Cochlear samples from a control (F) and an experimental (G) mouse stained for Myosin-VI, a pan-HC marker (yellow dashed line between the inner and outer hair cells is used to measure cochlear length). H Comparison of cochlear length between control and experimental mice at E16.5 and E18.5. At both ages, the cochlear duct is significantly shorter in experimental than in control mice. Data are presented as means ± SEM. *P <0.05, ***P <0.001. IHCs, inner hair cells; OHCs, outer hair cells; b, basal; m, middle; a, apical; ot, otocyst; hb, hindbrain; scale bars, 200 μm (B, C, F, G), and 20 μm (D, E).
