The 2021 Nobel Prize in Physiology or Medicine was awarded to two sensory neurobiologists and pain researchers, David Julius at the University of California in San Francisco and Ardem Patapoutian at Scripps Research in San Diego, for their discoveries of thermal and mechanical transducers [1] (https://www.nobelprize.org/prizes/medicine/2021/advanced-information/). As the Nobel Committee stated, “the question of how we sense the physical world through somatic sensation has fascinated humankind for millennia, however, the identity of the molecular transducers responsible for detecting and converting heat, cold and touch into nerve impulses in the sensory nervous system remained a mystery until the discoveries awarded with this year’s Nobel Prize” [1]. The pain research and neuroscience community is thrilled that the committee recognized the importance of these fundamental questions with this year’s Physiology or Medicine prize.
Capsaicin, TRPV1, and the Sensation of Noxious Heat
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is an active component of chili peppers. As a chemical irritant to animals and humans, capsaicin produces intense pain when applied to the skin. It also produces the burning sensation we experience when we eat spicy foods. The compound was first extracted in impure form in 1816 (Fig. 1), and was initially called "capsicin", after the plant genus Capsicum, which includes members such as the chili pepper and bell pepper. Capsaicin was instrumental to the work of Julius and this year’s Nobel Prize in Physiology or Medicine. A PubMed search for the keyword “capsaicin” produced 15,769 results, including 358 publications in 1996, a year prior to the cloning of the capsaicin receptor. As one of the most useful research tools, capsaicin has been a focus of pain research for several decades. Capsaicin specifically activates nociceptors, the primary sensory neurons that sense pain, via the activation of unmyelinated and slow-conducting C-fibers. In 1983, Fitzgerald reviewed early research on capsaicin and sensory neurons in the journal Pain. In 1988, Wood and coworkers revealed capsaicin-induced ion fluxes in dorsal root ganglion (DRG) neurons. In 1990, Szallasi and Blumberg demonstrated specific binding of resiniferatoxin (RTX), an ultrapotent capsaicin analog, by DRG membranes. In 1996, Levine and coworkers showed that noxious heat activated ion channels in sensory neurons. However, it remained unclear whether the transduction of heat and thermal energy required specific ion channels.
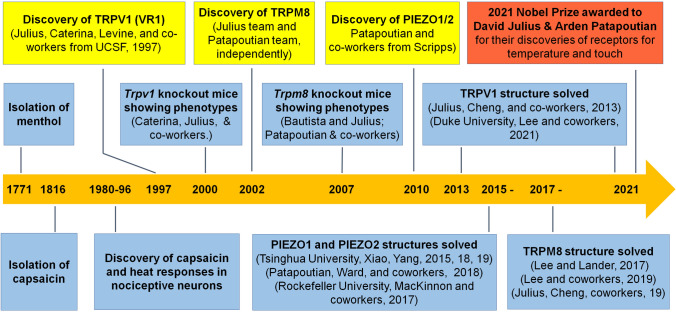
Fig. 1.
Time line and milestones of major discoveries and events.
Together with a postdoctoral fellow, Michael Caterina, now the Director of the Pain Research Center at Johns Hopkins University Medical School, Julius conducted unbiased functional screening to hunt for the capsaicin receptor. They tested the hypothesis that capsaicin sensitivity is conferred on cells that are normally insensitive to capsaicin if a single gene, encoding the capsaicin receptor, is expressed by cells. To identify this putative gene, Julius and coworkers created a cDNA library from rodent DRGs known to contain capsaicin-responsive sensory neurons. Using this expression cloning strategy together with calcium imaging, Julius’s team isolated a functional cDNA encoding a capsaicin receptor from sensory neurons [2]. This receptor was identified as a cation channel that is activated by increases in temperature in the noxious range. Interestingly, this receptor was found to be structurally related to members of the transient receptor potential (TRP) family of ion channels [2]. Jon Levine’s pain research group at UCSF was also involved in this seminal study published in Nature in 1997. The discovery of the capsaicin vanilloid receptor (VR1), later renamed TRPV1 (vanilloid subtype 1), is one of the most important breakthroughs in pain research. In the last 24 years, this study has been cited over 9100 times in Google Scholar and 6,112 times (with an average of 245 citations per year) in SCI.
The discovery of TRPV1 also led to the identification of other related temperature and/or pain sensing channels (e.g., TRPV2, TRPV3, and TRPA1). In 2000, Julius’ team published the finding of Trpv1 knockout mice, demonstrating the necessity for TRPV1 in not only the capsaicin response but also nociception [3]. In the same year, a British group in London (Davis et al., 2000) confirmed that TRPV1 is required for the development of carrageenan-induced inflammatory pain (heat hyperalgesia). Davis’ study also showed the acute thermal pain was not affected in Trpv1-null mice. Later, Voets, Vriens, and coworkers found that detection of noxious heat in mice depends on the collaborative action of three different types of TRP channel: TRPV1, TRPM3, and TRPA1. In the last two decades, TRPV1 has been extensively used as a marker to define C-fiber and peptidergic nociceptors. Further studies have revealed that TRPV1 not only regulates temperature but is also a general integrator of nociceptive signaling, as multiple inflammatory and pain mediators can cause TRPV1 activation or sensitization. Under chronic inflammatory pain conditions, TRPV1 is upregulated by nerve growth factor through p38 MAP kinase and undergoes axonal transport to the skin nerve terminals to mediate heat hyperalgesia [4]. Subsequent studies from multiple labs in China also demonstrated important roles of TRPV1 in inflammatory pain and cancer pain, as well as in substance P/NK-1 and CDK signaling in nociceptive sensory neurons. Recent studies have also demonstrated that TRPV1 contributes to different modalities of pain, including mechanical pain, induced by pro-inflammatory cytokines such as IL-17 [5]. TRPV1 also plays an important role in regulating itch (pruritus) [6]. Apart from the skin nerve terminals, TRPV1 in the central terminals of nociceptors in the spinal cord also plays an active role in regulating nociceptive synaptic transmission by inducing the release of pain-inducing neurotransmitters, such as glutamate and the neuropeptides substance P and CGRP.
Julius has also made another seminal contribution to structural biology of TRP channels, brought about by cryo-electron microscopy (Cryo-EM). He teamed up with his UCSF colleague, Yifan Cheng, a structural biophysicist who is a pioneer in the development of the Cryo-EM method. Together, they produced an extraordinarily detailed architecture of the TRPV1 ion channel [7]. The double knot toxin and RTX binding causes dilation of the selectivity filter and the intracellular gate, formed by the S6 transmembrane helices, leading to channel opening. In 2021, Lee and coworkers visualized heat-dependent opening of TRPV1 in the presence of capsaicin using Cryo-EM [8] (Fig. 2A). Heat-dependent TRPV1 opening involves stepwise conformational changes: global changes followed by rearrangements of the outer pore, leading to gate opening. Functional and structural analyses have identified an interactive network directly involved in heat sensing.
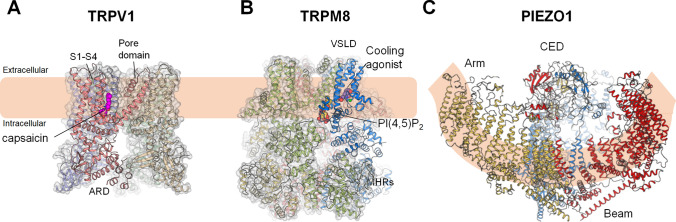
Fig. 2.
Structures of TRPV1 (A), TRPM8 (B), and PIEZO1 (C). Cryo-EM structures of receptors for temperatures (TRPV1 and TRPM8) and touch (PIEZO1). A Structure of the capsaicin and heat receptor TRPV1 in the heat-activated open state (PDB 7LPE). Capsaicin (magenta spheres) binds to the vanilloid binding pocket. B Structure of the menthol and cold sensing TRPM8 channel (PDB 6NR3). The membrane lipid PI(4,5)P2 (yellow spheres) and the cooling agonist (purple spheres) binding sites are located at a membrane interfacial cavity and at the voltage-sensor-like domain (VSLD), respectively. This strategic design principle of ligand recognition illuminates the synergistic actions of lipid and agonist on the activation of the TRPM8 channel. C Structure of PIEZO1 shown from the extracellular side (left) and from the plasma membrane (right) (PDB 6B3R). Arm, CED, and Beam domains are shown as indicated.
Immediately after its discovery, the pain research field knew that TRPV1 had a great chance of being medically important for pain management. It is not surprising that TRPV1 has been a hot drug target, and numerous pharmaceutical companies have developed various TRPV1 inhibitors. The drug discovery efforts have had disappointing results in clinical trials, in part due to the fever-inducing side-effects of TRPV1 antagonists [9]. Nevertheless, the clinical studies do support a critical role of TRPV1 in body temperature regulation in humans. On the other hand, TRPV1 agonists such as capsaicin cream have been used to treat neuropathic pain, and RTX is being evaluated in phase III trials for chronic pain associated with cancer and knee arthritis. The TRPV1 channel has also been used to deliver local anesthetics such as the sodium channel blocker QX-314. Woolf, Bean, and coworkers demonstrated that this strategy can selectively block the function of C-fibers and C-fiber-mediated pain [10].
Menthol, TRPM8, and the Sensation of Cold
Like capsaicin, menthol is a natural compound obtained from the oils of corn mint, peppermint, and other mints. In contrast to capsaicin, menthol elicits a cooling sensation and is widely used to relieve minor throat irritation. Menthol is known to have analgesic, anti-pruritic, and anti-cough effects, and has been used in Eastern medicine for 2000 years. In the West, menthol was isolated in 1771. Julius and Patapoutian independently used menthol in functional screens to examine how cells respond to cold. In 2002, Their efforts led to the discovery of another important ion channel named TRPM8 [11, 12]. In a heterologous expression system, TRPM8 was activated by low temperatures at a range which humans perceive as innocuous cold. Further studies in Trpm8-deficient mice have demonstrated that cultured sensory neurons and intact sensory nerve fibers exhibit marked deficits in responses to cold stimuli [13]. Deletion of Trpm8 in knockout mice causes clear deficits in the sensation of innocuous cold (cooling), and in response to menthol, and acetone, but limited deficits in noxious cold sensation [13, 14]. TRPM8 also mediates cold-induced allodynia and cold-induced analgesia [14]. The discovery of TRPM8 as a cold sensor supported the central role of the TRP superfamily in the somatosensation of temperature and led to the identification of additional TRP channels involved in thermal sensation such as TRPM2 and TRPM3.
Seok-Yong Lee’s group in Duke University reported the structure of TRPM8 in 2017 in collaboration with the Lander group in Scripps Research Institute [15] (Fig. 2B). The structure provides the novel architecture of TRPM8, which is distinct from the previously reported TRP channels. Lee’s group subsequently solved the structures of TRPM8 in complex with cooling agents and the signaling lipid PI(4,5)P2, thereby illustrating the structural basis of chemically-induced cold sensation by the TRPM8 channel for the first time [16]. In 2019, Julius, Cheng, and coworkers also provided structural insights into TRPM8 inhibition and desensitization (Fig. 1). Studies on the interactions of TRPM8 with its modulators, together with simulation studies, will lead to the development of more selective and potent TRPM8 agonists that have multiple benefits for the control of cough, ocular pain, and dry eye disease.
PIEZO and Pressure Sensation
While searching for thermal sensors, Patapoutian was also looking for mechanoreceptors, molecules activated by mechanical force (pressure). Together with postdoctoral fellow Bertrand Coste, the team developed a novel screening approach to find potential receptors for mechanosensation in mammals. The team first identified an intrinsically mechanosensitive cell line (Neuro2A) that can generate inward current by using brief and rapid indentation of the plasma membrane, and then hunted for genes responsible for the currents using siRNA knockdown. In 2010, this bold effort led to the discovery of two novel ion channels, named PIEZO1 and PIEZO2 [17] (Fig. 1), which exhibit distinct expression patterns: PIEZO2 is expressed in primary sensory neurons, whereas PIEZO1 is expressed by non-neuronal cells. In 2012, the team further demonstrated that PIEZO proteins are pore-forming mechanical channels. In a later study in 2014, Patapoutian’s team engineered mice that lacked Piezo2 in both Merkel cells and adult sensory neurons. These mutant mice exhibited a profound loss of touch sensation. Through collaboration with multiple pain research groups world-wide, they found that touch and pain sensation may be separable, suggesting that additional ion channels must account for noxious (painful) mechanosensation [18]. It is well known that under pathological conditions such as nerve injury, normally innocuous stimulation such as light touch can be painful, producing mechanical allodynia or tactile allodynia. Patapoutian’s team demonstrated that mechanical allodynia (punctate and dynamic allodynia) after nerve injury was absent in Piezo2-deficient mice. It is also well-established in pain research that large A-fiber stimulation (e.g., massage) inhibits pain through “Gate control” in the spinal cord. Thus, PIEZO activation may also suppress acute pain, and loss of PIEZO2 in sensory neurons is associated with increased mechanical sensitivity in mice. The PIEZO2 channel also regulates mechanical itch (alloknesis) through Merkel cell signaling in skin diseases associated with chronic itch. Importantly, humans with loss-of-function mutations in PIEZO2 also display profound deficits in touch sensation, including texture discrimination and hair deflection, as well as tactile and vibration sensitivity [19].
From the view of molecular biology, PIEZO proteins represent a new class of mechanosensitive channels and do not have any similarity to previously characterized ion channel families. As the largest transmembrane ion channel identified so far, PIEZO contains 2,500 amino-acids and a 38-transmembrane helix. The PIEZO1 structure was first revealed in 2015 [20] by a group of investigators at Tsinghua University, where Cryo-EM has been extensively used to solve a large variety of structures of important proteins. Bailong Xiao, a biochemist in Tsinghua and a former postdoctoral researcher in Patapoutian’s lab, has solved the structures of both PIEZO1 and PIEZO2. Patapoutian’s group and Roderick Mackinnon’s group at Rockefeller University also solved the PIEZO1 structure independently (Fig. 1). Cryo-EM revealed the PIEZO channel as a homotrimeric structure with a central ion-conducting pore and three peripheral large propeller-shaped blades that are responsible for mechanosensing (Fig. 2C).
Summary and Future Directions
The discoveries of David Julius and Ardem Patapoutian have fundamentally changed our understanding of sensory biology, as mutations of these ion channels are associated with defects in human senses and physiological functions. As summarized by the Nobel Committee, “TRPV1 and PIEZO2 channels endow us with the sensation of temperature, heat pain, touch and proprioception” [1]. TRPV1 plays critical roles in regulating core body temperature, inflammatory pain, neuropathic pain, visceral pain, and protective reflexes, while PIEZO2 is important for mechanical pain, urination, respiration, blood pressure, and skeletal remodeling [1]. There are still outstanding questions, however. First, there are additional thermal and pressure receptors and transducers in sensory neurons and non-neuronal cells, and investigating their interactions with TRP and PIEZO channels in different cell types is of great interest. Second, temperature sensation by TRP channels is not fully understood at the molecular/structural levels. Third, specific types of nerve fibers (primary afferents) and the central neural circuits for the conduction of temperature and pressure sensation are not fully established, although great progress has been made in this area. Finally, novel therapeutics are awaiting development for the treatment of a wide range of disease conditions, including chronic pain and chronic itch, both of which are critically regulated by TRP channels [6]. Addressing these important questions will offer new directions for the sensory neurobiology and pain research community.
Acknowledgements
We thank Ying Yin from Seok-Yong Lee’s lab for help with the preparation of Fig. 2.
Conflict of interest
The authors have no competing financial interest in this study.
References
- 1.Ernfors P, Manira AE, Svenningsson P. Scientific background: Discoveries of receptors for temperature and touch. The Nobel Assembly at Karolinska Institute 2021. https://www.nobelprize.org/prizes/medicine/2021/advanced-information/
- 2.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 4.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/S0896-6273(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 5.Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron. 2021;109:2691–2706.e5. doi: 10.1016/j.neuron.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and itch by TRP channels. Neurosci Bull. 2018;34:120–142. doi: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon DH, Zhang F, Suo Y, Bouvette J, Borgnia MJ, Lee SY. Heat-dependent opening of TRPV1 in the presence of capsaicin. Nat Struct Mol Biol. 2021;28:554–563. doi: 10.1038/s41594-021-00616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 11.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 12.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 13.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 14.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Yin Y, Wu M, Zubcevic L, Borschel WF, Lander GC, Lee SY. Structure of the cold- and menthol-sensing ion channel TRPM8. Science. 2018;359:237–241. doi: 10.1126/science.aan4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Y, Le SC, Hsu AL, Borgnia MJ, Yang HH, Lee SY. Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science. 2019;363:6430. doi: 10.1126/science.aav9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516:121–125. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, et al. The role of PIEZO2 in human mechanosensation. N Engl J Med. 2016;375:1355–1364. doi: 10.1056/NEJMoa1602812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527:64–69. doi: 10.1038/nature15247. [DOI] [PubMed] [Google Scholar]