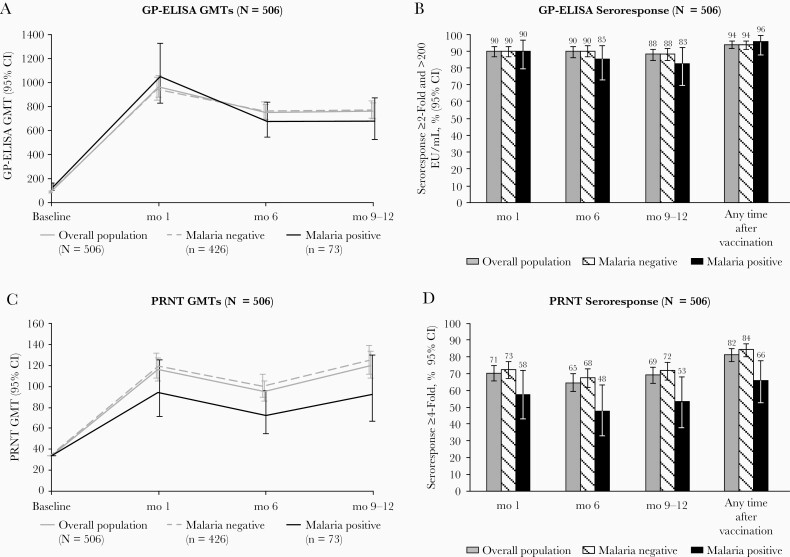
Figure 1.
Antibody responses through months 9–12 after vaccination with recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein (GP) vaccine, by baseline asymptomatic malaria infection, in the immunogenicity substudy of the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE). A, B, GP enzyme-linked immunosorbent assay (ELISA) results. C, D, Plaque reduction neutralization test (PRNT) results, including geometric mean titers (GMTs) (A, C) and seroresponse percentages (B, D). A ≥2-fold increase from baseline along with ≥200 ELISA units (EU)/mL was the definition that best differentiated vaccine from placebo recipients in the Partnership for Research on Ebola Vaccines in Liberia (PREVAIL) clinical trial. Abbreviation: CI, confidence interval.