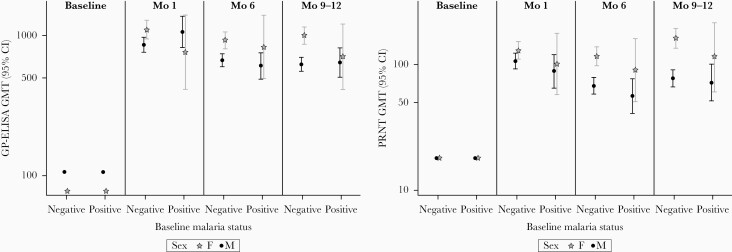
Figure 2.
Antibody responses by baseline malaria parasitemia (present vs absent) and by sex, at baseline and 1, 6 and 9–12 months after vaccination with recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein (GP) vaccine. Results from the immunogenicity substudy of the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) and based on analysis of covariance adjusted for the GP-ELISA GMTs and baseline malaria status by visit and sex. Abbreviations: CI, confidence interval; GMT, geometric mean titer; PRNT, plaque reduction neutralization test.