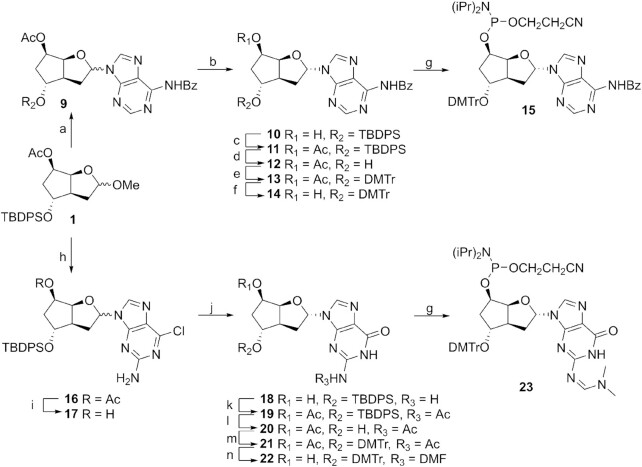
Figure 3.
Synthesis of purine building blocks. (a) N6-Benzoyladenine, BSA, TMSOTf, MeCN, 70°C, 20 min, 64%; (b) NaOH, THF/MeOH/H2O, 0°C, 20 min, 51% α-anomer, 18% β-anomer; (c) Ac2O, DMAP, DCM, rt, 18 h, 90%; (d) TBAF, THF, rt, 3.5 h, 90%; (e) DMTr-Cl, pyridine, rt, 24 h, 89%; (f) NaOH, THF/MeOH/H2O, 0°C, 30 min, 94%; (g) 2-cyanoethyl N,N,N′,N′-tetraisopropylphosphordiamidite, ETT, DCM, rt, 1 h, 77% for 15, 50 min, 67% for 23; (h) 2-amino-6-chloropurine, BSA, TMSOTf, MeCN, 55°C, 50 min, 77%; (i) NaOH, THF/MeOH/H2O, 0°C, 20 min, 85%; (j) TBD, 3-hydroxypropionitrile, DCM, 48 h, 87%; (k) Ac2O, DMAP, DCM, rt, 48 h, 76%; (l) TBAF, THF, rt, 4 h, 87%; (m) DMTr-Cl, pyridine, rt, 48 h, 99%; (n) (i) K2CO3, MeOH, rt, 7 h, (ii) N,N-dimethylformamide dimethylacetal, DMF, 55°C, 2 h, 77%.