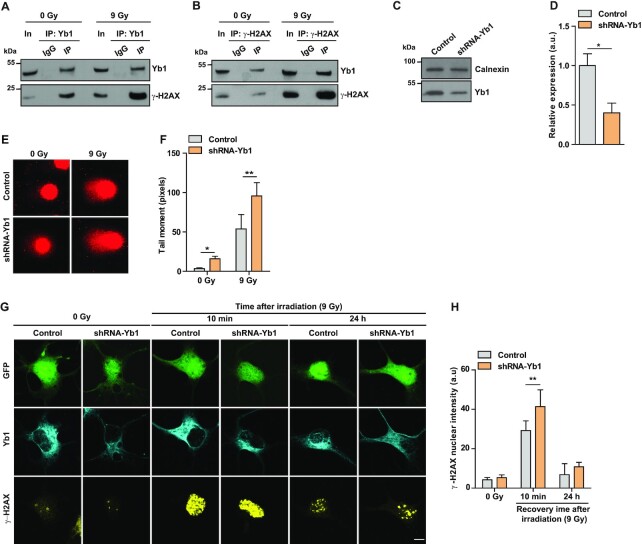
Figure 7.
Yb1 knockdown results in DNA damage defects in motoneurons. (A, B) Immunoprecipitation of Yb1 (A) or γ-H2AX (B) performed on whole lysates of non-irradiated (0 Gy) or irradiated (9 Gy) NSC-34 cells. Proteins were analyzed by western blot with antibodies against γ-H2AX and Yb1. In, input; IP, immunoprecipitation. (C) Western blot analysis of Yb1 levels in cultured motoneurons transduced with control or shRNA-Yb1. Calnexin was used as loading control. (D) Quantification of western blot data shown in (C). Data are mean ± SD (n = 3 independent experiments). Statistical analysis was performed using unpaired t-test; *P ≤ 0.05. (E) Representative images of alkaline comet assay performed on control and shRNA-Yb1-transduced motoneurons under non-irradiated conditions (0 Gy) or after exposure to γ-irradiation (9 Gy). (F) Quantification of comet mean tail moments. Data are mean ± SD (n = 3 independent experiments; N = 31 nuclei for control (0 Gy), N = 34 nuclei for control (9 Gy), N = 33 nuclei for shRNA-Yb1 (0 Gy) and N = 33 nuclei for shRNA-Yb1 (9 Gy)). Statistical analysis was performed using two-way ANOVA followed by Bonferroni post-hoc test; *P ≤ 0.05, **P ≤ 0.01. (G) Representative images of γ-H2AX immunofluorescence staining of control and shRNA-Yb1-transduced motoneurons under non-irradiated conditions (0 Gy) or after exposure to γ-irradiation (9 Gy) followed by the indicated recovery times. EGFP was used as a marker to identify transduced cells. Scale bar: 5 μm. (H) Quantification of nuclear γ-H2AX immunostaining in (G). Data are mean ± SD (n = 4 independent experiments; N = 42 nuclei for control (0 Gy), N = 40 nuclei for control (9 Gy,10 min recovery), N = 18 nuclei for control (9 Gy, 24 h recovery), N = 34 nuclei for shRNA-Yb1 (0 Gy), N = 43 nuclei for shRNA-Yb1 (9 Gy, 10 min recovery) and N = 31 nuclei for shRNA-Yb1 (9 Gy, 24 h recovery)). Statistical analysis was performed using two-way ANOVA followed by Bonferroni post-hoc test; **P ≤ 0.01.