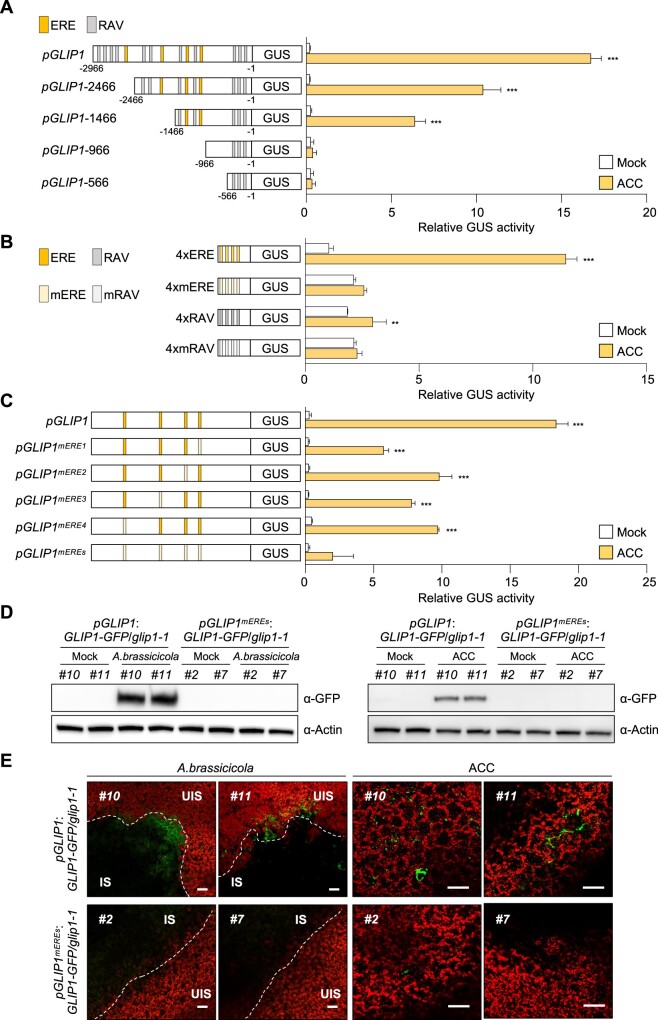
Figure 1.
ERE is the essential regulatory element in the GLIP1 promoter. A, GUS reporter assays showing ACC-induced expression of the GUS reporter gene driven by the full-length and truncated GLIP1 promoters. The left part illustrates deletions of the GLIP1 promoter. The ERE and RAV elements in the GLIP1 promoter are boxed in yellow and gray, respectively. B, GUS reporter assays showing ACC-induced expression of the GUS reporter gene driven by synthetic promoters of 4xERE and 4xRAV, and their mutant versions 4xmERE and 4xmRAV. The left illustrates synthetic promoters. C, GUS reporter assays showing ACC-induced expression of the GUS reporter gene driven by the GLIP1 promoters with individual or all ERE mutations. The left illustrates ERE mutations of the GLIP1 promoter. D, Immunoblot analysis of GLIP1-GFP expression in A. brassicicola- and ACC-treated pGLIP1:GLIP1-GFP glip1 and pGLIP1mEREs:GLIP1-GFP glip1 plants. Protein extracts were subjected to immunoblotting with anti-GFP and anti-Actin antibodies. Actin levels served as a control. E, Confocal images of GLIP1-GFP expression in A. brassicicola- and ACC-treated pGLIP1:GLIP1-GFP glip1 and pGLIP1mEREs:GLIP1-GFP glip1 plants. Bars, 100 μm. Two independent transgenic lines were used in all experiments. In (A–C), transfected protoplasts were treated with mock (water) and ACC (200 μM) for 6 h. Values represent means ±sd (standard deviation) (n = 3 biological replicates). Asterisks indicate significant differences from mock treatment as determined by one-way ANOVA with Tukey test (**P < 0.01; ***P < 0.001). In (D and E), 6-week-old plants were treated with ACC (1 mM) for 6 h or with 5 µL droplets of A. brassicicola spore suspensions (5 × 105 spores mL−1) for 2 d. IS, infected site; UIS, uninfected site.