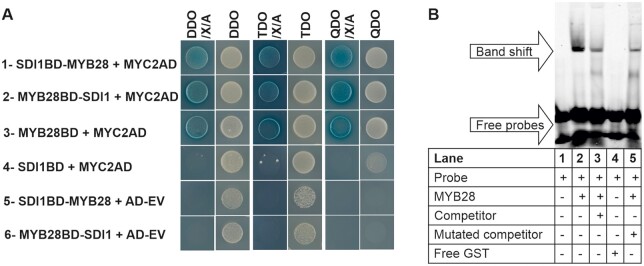
Figure 6.
Investigation of ternary protein complex formation among SDI1, MYB28, and MYC2 and binding activity of MYB28 to SESA4 promoter. A, Panels 1 and 2 demonstrate the positive interaction among SDI1, MYB28, and MYC2 in Y3H screenings. In panel 1 SDI1BD-MYB28 expressed SDI1 fused to the DNA-BD (Gal4 DNA-binding domain) as well as MYB28 expressing as the bridging protein. In panel 2, MYB28BD-SDI expressed MYB28 fused to the DNA-BD domain and SDI1 as the bridging protein. MYC2AD was used as prey expressing MYC2 fused to the activation domain (AD). Cotransformations of prey empty vector (AD-EV) with pBridge baits were performed as negative controls in the last two panels. Y2H assays were performed as in panels 3 and 4 by co-transformation of the respective prey and bait constructs grown on dropout plates with or without X-α-Gal (X) and Aureobasidin (A). The double (DDO), triple (TDO), and quadruple (QDO) dropout media are described in the “Material and methods” section. B, EMSA shows the binding activity of MYB28 to the promoter of At2S4 or SESA4. The probe sequences were designed as described in the “Material and methods”. The presence or absence of the reagents in each lane is indicated with (+) and (−), respectively. Adding the unlabeled competitor in molar excess reduced the signal intensity. However, we could not confirm the specificity of the interaction between the selected MYB cis-element in the promoter of SESA4 and MYB28 because adding the mutated version of the unlabeled probe diminished the binding intensity.