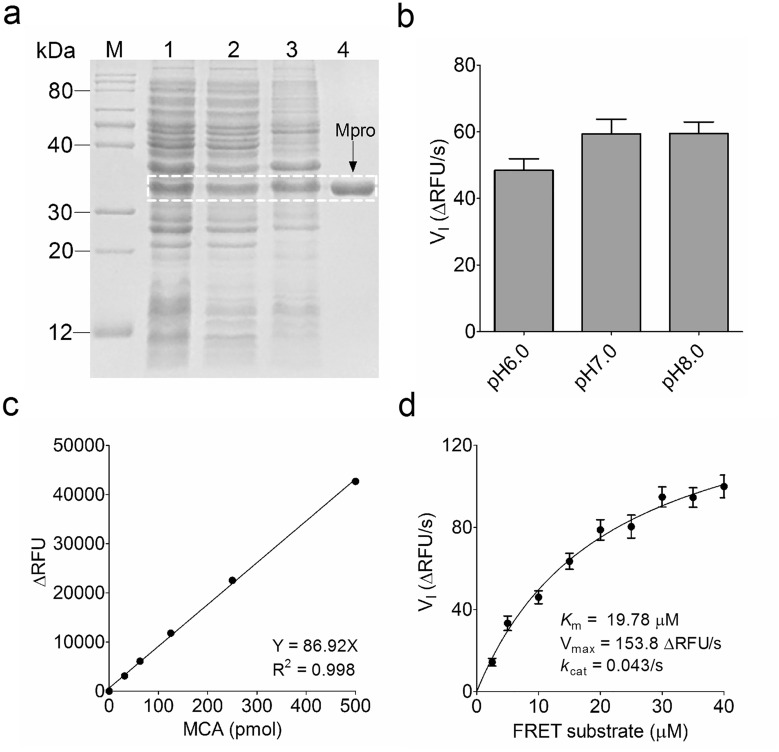
Fig. 1.
Preparation and characterization of SARS-CoV-2 main protease (Mpro). a Expression and purification of SARS-CoV-2 Mpro. The highly active Mpro was expressed in E. coli Rosetta (DE3) cells as the soluble protein after induction with 0.2 mM IPTG at 30 °C for 8 h. A HisTrapTM chelating column was used to purify polyhistidine-tagged Mpro from E. coli cell extracts, and then the purity of purified Mpro was analyzed using SDS-PAGE. M: protein marker; 1: total cell extracts after IPTG induction; 2: supernatant of the cell lysate; 3: precipitated Mpro in 25% saturated ammonium sulfate solution; 4: purified Mpro band (34 kDa). b Reaction buffer optimization. The reaction mixture containing 0.25 µM Mpro and 10 µM FRET substrate was incubated in the indicated three buffers, and the change of RFU value was continuously recorded by a microplate reader (BioTek) using a FRET assay. The initial velocity (VI) of the proteolytic activity was calculated by a linear regression for the first 30 s of the kinetic progress, and the curve was plotted in GraphPad Prism 5.0. c Plotting the MCA standard curve to convert RFU value to the amount of the cleaved FRET substrate (pmol) using the derived equation. MCA: 7-methoxycoumarin-4-acetic acid. d Calculation of Mpro kinetic constant Km and kcat values in the FP assay buffer. The Michaelis-Menten equation of Mpro proteolytic kinetics was plotted using GraphPad Prism 5.0 according to the VI, and then Km, Vmax and kcat values were calculated