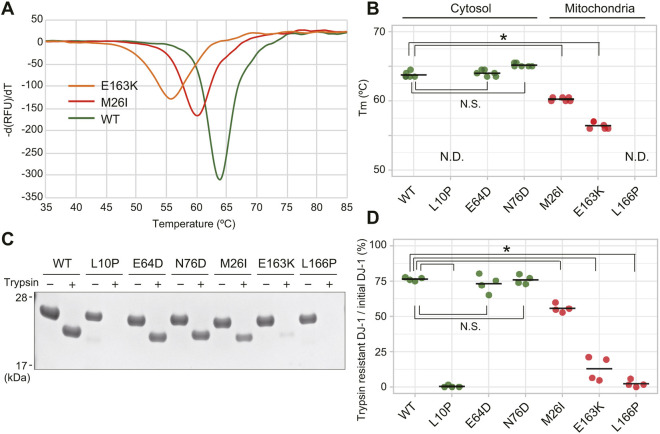
Fig. 2.
Folding instability of DJ-1 pathogenic mutants determines their mitochondrial localization. (A,B) The melting temperatures (Tm) of N-terminal 6×His-tagged DJ-1 proteins were measured via a thermal shift assay. Representative thermal spectra are shown in A, and averaged Tm with individual data points of three independent experiments are shown in B. Mitochondria-localized pathogenic DJ-1 mutants are characterized by a higher Tm. No Tm could be determined (N.D.) for the L10P mutation, suggesting the absence of any structure. (C,D) Folding stability of DJ-1 pathogenic mutants under physiological temperature (37°C) assessed via trypsin digestion. Recombinant 6×His-DJ-1 proteins with the indicated pathogenic mutations were incubated in the presence or absence of trypsin. (C) Representative immunoblotting data of two independent experiments are shown. (D) Degree of trypsin susceptibility after incubation for 22 h. Individual data points from two independent experiments are shown. *P<0.01, N.S., not significant (one-way ANOVA with Sidak's correction).