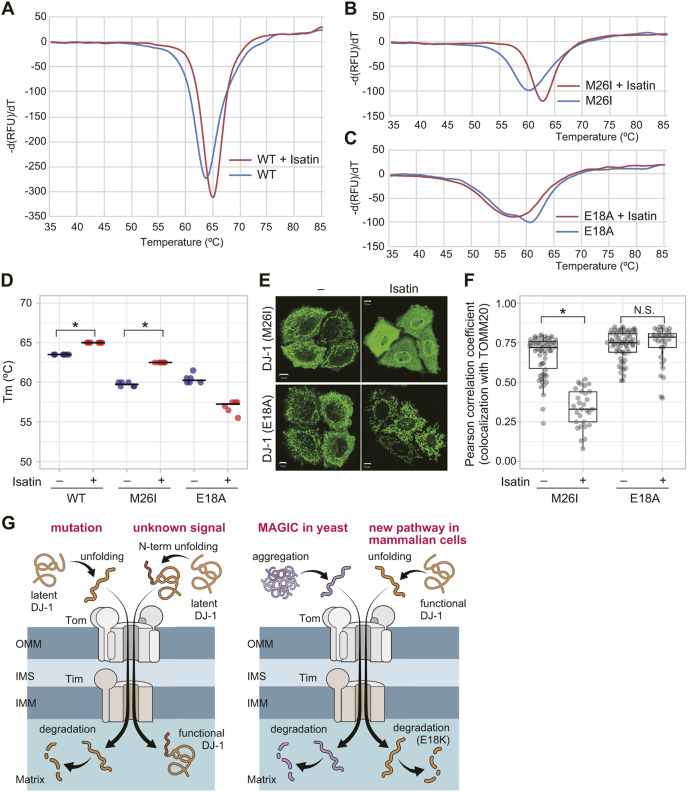
Fig. 8.
DJ-1 stabilization reverses mitochondrial localization of DJ-1 mutants. (A–C) Representative thermal shift spectra for WT DJ-1 (A), M26I (B), or E18A (C) mutant in the presence of isatin. Isatin stabilizes and increases the melting temperature (Tm) of the M26I mutant but does not stabilize the E18A mutant. (D) Quantified data of the thermal shift assay are shown. Individual data points of three independent experiments are shown. *P<0.01 compared to untreated control (one-way ANOVA with Sidak's correction). (E) The subcellular localization of E18A and M26I mutants in the absence or presence of isatin when expressed in DJ-1-knockout HeLa cells. Representative images of two independent experiments are shown. Scale bars: 10 µm. (F) The colocalization of DJ-1 M26I or E18A mutants with TOMM20 following isatin treatment was calculated as a Pearson correlation coefficient in individual cells. *P<0.01, N.S., not significant 01 compared to untreated control (one-way ANOVA with Sidak's correction). Isatin stabilization of the DJ-1 M26I mutant restores its cytoplasmic localization, whereas the isatin-insensitive E18A mutant remains localized in mitochondria. Box plots are as described in the Materials and Methods section. (G) Two possible schematic models for DJ-1 translocation into mitochondria. Left panel; restricted unfolding by unknown signal and subsequent mitochondrial translocation is essential for the genuine function of DJ-1, and that the mitochondria-localized DJ-1 mutants reflect this phenomenon. Right panel; mitochondrial localization of DJ-1 reflects an undetermined mitochondria-based quality control system for cytoplasmic proteins like “MAGIC” in yeast cells. See text for details.