Abstract
Objectives
Interleukin 11 (IL11) is highly upregulated in skin and lung fibroblasts from patients with systemic sclerosis (SSc). Here we tested whether IL11 is mechanistically linked with activation of human dermal fibroblasts (HDFs) from patients with SSc or controls.
Methods
We measured serum IL11 levels in volunteers and patients with early diffuse SSc and manipulated IL11 signalling in HDFs using gain- and loss-of-function approaches that we combined with molecular and cellular phenotyping.
Results
In patients with SSc, serum IL11 levels are elevated as compared with healthy controls. All transforming growth factor beta (TGFβ) isoforms induced IL11 secretion from HDFs, which highly express IL11 receptor α-subunit and the glycoprotein 130 (gp130) co-receptor, suggestive of an autocrine loop of IL11 activity in HDFs. IL11 stimulated ERK activation in HDFs and resulted in HDF-to-myofibroblast transformation and extracellular matrix secretion. The pro-fibrotic action of IL11 in HDFs appeared unrelated to STAT3 activity, independent of TGFβ upregulation and was not associated with phosphorylation of SMAD2/3. Inhibition of IL11 signalling using either a neutralizing antibody against IL11 or siRNA against IL11RA reduced TGFβ-induced HDF proliferation, matrix production and cell migration, which was phenocopied by pharmacological inhibition of ERK.
Conclusions
These data reveal that autocrine IL11-dependent ERK activity alone or downstream of TGFβ stimulation promotes fibrosis phenotypes in dermal fibroblasts and suggest IL11 as a potential therapeutic target in SSc.
Keywords: systemic sclerosis, IL11, IL11RA, TGFβ2, antibody therapy, fibrosis, neutralizing antibody
Rheumatology key messages
Serum IL11 levels are elevated in patients with diffuse systemic sclerosis compared with healthy controls.
IL11-dependent ERK signalling is required for TGFβ to elicit a profibrotic response in dermal fibroblasts.
Neutralizing IL11 antibodies block TGFβ2-driven myofibroblast activation in systemic sclerosis fibroblasts.
Introduction
Dermal fibrosis is a pathological hallmark of chronic inflammatory skin conditions such as SSc, atopic dermatitis and psoriasis. In SSc, fibrosis also occurs in visceral organs, notably the lung, and is accompanied by proliferative vasculopathy [1, 2] and inflammation [3].
TGFβ family proteins are the quintessential mediators of fibroblast activation across organs and diseases, including SSc [4]. The TGFβ2 isoform is notably upregulated in fibroblasts and skin biopsies from patients with SSc as compared with healthy individuals, at both the RNA and the protein level [5, 6]. Thus, anti-fibrotic strategies for patients with SSc need to be carefully selected based on the ability to inhibit TGFβ2 signalling and those targeting integrin-mediated TGFβ activation may not be effective. Unfortunately, targeting TGFβ in general is associated with serious side effects, which have precluded the use of anti-TGFβ reagents in the clinic, to date [7, 8].
Interleukin (IL) 11 has recently been identified as a TGFβ-responsive profibrotic cytokine [9, 10], and its gene has been shown to be one of the most highly upregulated in fibroblasts from SSc lesional skin and in pulmonary fibroblasts of patients with interstitial lung disease due to SSc [6, 11]. Myofibroblasts are the major cell type that underlie fibrosis and secrete copious amounts of extracellular matrix while also being pro-inflammatory. Given that TGFβ-driven myofibroblast activation is IL11-dependent in fibroblasts from some tissues and stromal cells more generally [12], we set out to characterize IL11 as a potential fibrogenic factor in dermal fibroblasts from controls and patients with SSc.
Methods
Detailed methods are provided in the supplementary data file that accompanies this paper, which includes detailed methods on the following: patient cohort, cell culture, recombinant proteins and chemicals, sequencing of the IL11RA mutation, RNA sequencing, immunofluorescence staining, ELISA, immunoblotting, siRNA knockdown, RT-qPCR, high-content imaging assays, half-maximal inhibitory concentration measurement, migration assay and statistical analysis.
Results
IL11 levels are elevated in the serum of patients with early diffuse SSc
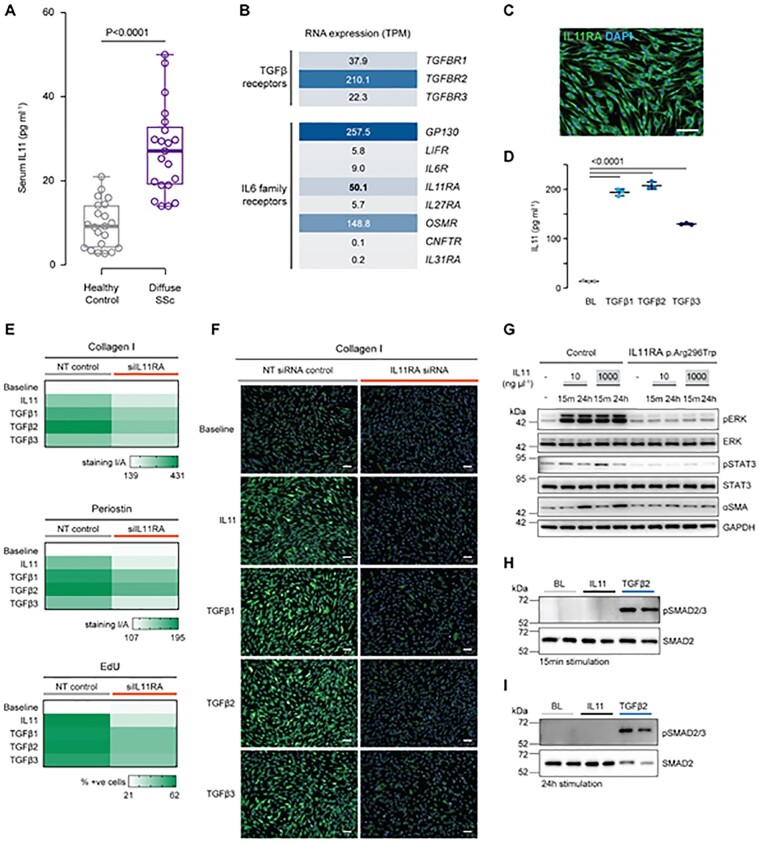
We measured circulating IL11 levels in serum from a cohort of 21 patients with early diffuse SSc (<2 years from onset of non-Raynaud’s symptoms) by ELISA (Human Quantikine IL11, R&D Systems, Minneapolis, MN, USA) and found that IL11 levels were significantly elevated in the serum of SSc patients compared with sex-matched healthy controls (Fig. 1A, mean SSc: 27.2 (10.5) pg/ml; mean controls: 9.6 (5.5) pg/ml; P < 0.001; further details in Supplementary Table S1 and Supplementary Fig. S1, available at Rheumatology online). Thus IL11 is not only highly expressed in skin and lung fibroblasts from patients with SSc [6, 11] but is also systematically elevated.
Fig. 1.
IL11 levels are increased in the serum of patients with systemic sclerosis and IL11 has SMAD-independent profibrotic activity in human dermal fibroblasts
(A) Serum IL11 levels in patients with diffuse SSc and sex-matched healthy controls. Data: n = 21 per group (Supplementary Table S1, available at Rheumatology online), whiskers from min. to max. value, line at median. Statistics: two-tailed t-test. (B) RNA-seq of primary human dermal fibroblasts (n = 4). RNA expression values are shown as transcripts per million (TPM) for receptors of the TGFβ and IL6 cytokine family. (C) IL11RA staining in unstimulated HDFs (scale bar, 100 µm). (D) IL11 secretion by HDFs stimulated with three TGFβ isoforms (5 ng/ml) for 24 h. Data (n = 3): mean (s.d.). Statistics: one-way ANOVA, Dunnett’s multiple testing correction. (E) Quantification of signal intensity/area (collagen I, periostin) and percentage of EdU-staining positive HDFs subjected to siRNA knockdown for IL11RA and stimulation with TGFβ1, TGFβ2, TGFβ3 or IL11(5 ng/ml) for 24 h. NT: non targeting siRNA control. (F) Collagen I staining representative images (periostin and EdU staining images are shown in Supplementary Fig. S2E, available at Rheumatology online) of HDFs that had been subjected to siRNA knockdown for IL11RA and stimulation with the indicated factors for 24 h. (G) Western blots assessing protein levels of phospho(p)ERK, ERK, pSTAT3, STAT3, αSMA, GAPDH in primary dermal fibroblasts of control and from an individual carrying a homozygous germline loss-of-function p.Arg296Trp mutation in IL11RA. (H and I) Western blot of SMAD2 and pSMAD2/3 expression in HDFs following stimulation with TGFβ2 or IL11 (5 ng/ml; 15 min or 24 h). EdU: 5-ethynyl-2′-deoxyuridine; ERK: extracellular signal-regulated kinase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; HDF: human dermal fibroblast; STAT: signal transducer and activator of transcription.
IL11 signalling drives HDF-to-myofibroblast transformation
To begin to examine whether IL11 plays a role in dermal fibroblast biology, we performed RNA-sequencing (RNA-seq) of primary human dermal fibroblasts (HDFs). Analyses revealed that IL11 receptor α-subunit (IL11RA) is one of the most highly expressed members of the IL6 family of receptors (Fig. 1B), which signal via gp130. TGFβ receptor (TGFβR) 2 transcripts were also highly expressed, whereas TGFβR1 and TGFβR3 RNA levels were less abundant (Fig. 1B). Immunofluorescence staining of HDFs revealed strong expression of IL11RA at the protein level (Fig. 1C).
We stimulated HDFs with TGFβ1, TGFβ2 or TGFβ3 and found that all isoforms induced IL11 secretion (Fig. 1D). These data provide evidence that HDFs are both a source and a target of IL11 and that IL11 signalling, perhaps in an autocrine mode, is related to TGFβ-stimulated effects in HDFs.
To assess whether IL11 secretion from HDFs following TGFβ stimulation was related to profibrotic phenotypes, we stimulated HDFs with IL11, TGFβ1, TGFβ2 or TGFβ3 in the presence of either non-targeting control siRNA or IL11RA siRNA (siIL11RA) (Fig. 1E-F; Supplementary Fig. S2A–E, available at Rheumatology online). All stimuli induced HDF proliferation and secretion of the extracellular matrix proteins collagen I and periostin. Inhibition of IL11 signalling by siIL11RA inhibited the profibrotic effects of not only IL11 but also all TGFβ isoforms. These data show that IL11 is a profibrotic factor in HDFs and that TGFβ-driven HDF activation is dependent, at least in part, on autocrine IL11 activity.
Dissection of signalling events downstream of IL11 activation in dermal fibroblasts
To investigate the signalling mechanisms underlying the profibrotic effects of IL11 in skin fibroblasts, we employed the use of HDFs obtained from an individual with Kreiborg–Pakistani syndrome (MIM: 614188). This individual lacks IL11 signalling due to a homozygous p.Arg296Trp loss-of-function mutation in IL11RA, which we confirmed by sequencing (Supplementary Fig. S2F, available at Rheumatology online) [13]. We compared these IL11RA mutant fibroblasts to a healthy age- and ethnically matched control and treated both control and mutant HDFs with either low dose/physiological (10 ng/ml) or high dose/supra-physiological (1000 ng/ml) recombinant human IL11 (Fig. 1G).
Consistent with data from fibroblasts from other organs, physiological concentrations (10 ng/ml) of IL11 maximally activated extracellular signal-regulated kinase (ERK) in control human HDFs at an early time point (15 min) and sustained this activation throughout the stimulation period (24 h). While IL11 has been shown to stimulate signal transducer and activator of transcription (STAT) in some contexts, we observed negligible phosphorylation of STAT3 with physiological IL11 levels and limited phosphorylation with a supraphysiological dose in HDFs (Fig. 1G). IL11-induced ERK and STAT3 phosphorylation were absent in IL11-stimulated HDFs from the IL11RA p.Arg296Trp mutant individual. IL11 stimulation of HDFs resulted in increased α-smooth muscle actin (αSMA) expression that was absent in IL11RA p.Arg296Trp HDFs (Fig. 1G).
We next assessed the potential dependency of the pro-fibrotic effects of IL11 on canonical TGFβ2 and/or SMAD2/3 activity. We observed that following stimulation of HDFs with IL11 there was no increase in TGFβ levels (Supplementary Fig. S2G and H, available at Rheumatology online) and no detectable SMAD2/3 phosphorylation at either early or late time points (Fig. 1H and I). These data are consistent with findings in cardiac fibroblasts [9] and rule out a substantive role for a TGFβ-dependent feed-forward loop downstream of IL11 activity in HDFs.
Inhibition of IL11 signalling blocks TGFβ2-stimulated dermal fibroblast- to-myofibroblast transformation
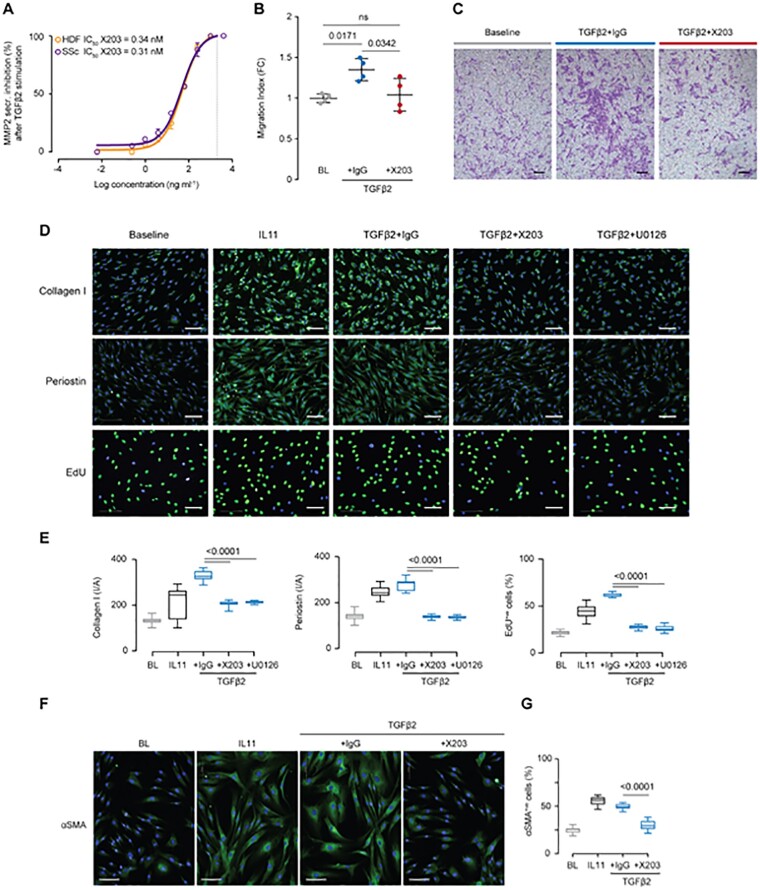
Next, we sought to understand whether the use of a neutralizing IL11 antibody (X203) [14] could block activation of HDFs. We first tested the efficacy of X203 in inhibiting the secretion of MMP2 from TGFβ2-stimulated HDFs [14]. X203 dose-dependently inhibited TGFβ2-induced MMP2 secretion by both primary HDFs (orange line) and SSc fibroblasts (purple line) with half-maximal inhibitory concentration (IC50) of 0.34 nM and 0.31 nM, respectively (Fig. 2A).
Fig. 2.
IL11 antibodies block TGFβ2-driven activation of skin fibroblasts from SSc patients and controls
(A) Dose–response curve and X203 IC50 value in inhibiting MMP2 secretion by HDFs and SSc dermal fibroblasts stimulated with TGFβ2. (X203 serial 4-fold dilution from 4 µg/ml to 61 pg/ml; line at x = 2 µg/ml; total n = 2 per group.) (B) Transwell migration indices of HDFs following 24 h stimulation with TGFβ2 (5 ng/ml) and in the presence of IgG or X203 (2 μg/ml). Data: n = 4, mean with s.d. Statistics: one-way ANOVA, Tukey’s multiple testing correction. (C) Migration assay representative images; scale bar, 150 µm. (D) Representative images of collagen I, periostin and EdU stainings of HDFs, following a 24 h stimulation with IL11 (5 ng/ml), or a combination of TGFβ2 (5 ng/ml) and IgG (2 μg/ml), IL11 neutralizing antibody (X203, 2 μg/ml) or ERK inhibitor (U0126, 10 μM); scale bar, 100 µm. (E) Quantification of signal intensity/area (collagen I, periostin) and percentage of EdU-staining positive cells relative to experiment in (D). Statistics: one-way ANOVA, Tukey’s multiple testing correction. (F and G) Representative images and quantification of percentage of αSMA-staining positive cells following a 24 h stimulation of SSc fibroblasts with the indicated factors. Data: whiskers from min. to max. value, line at median. Statistics: one-way ANOVA, Tukey’s multiple testing correction. αSMA: α-smooth muscle actin; EdU: 5-ethynyl-2′-deoxyuridine; ERK: extracellular signal-regulated kinase; HDF: human dermal fibroblast.
In migration assays, we found that IL11 or TGFβ2 promoted HDF migration and that X203 reduced the migratory capacity of TGFβ2-stimulated HDFs as compared with control (Fig. 2B and C and Supplementary Fig. S3A and B, available at Rheumatology online). Furthermore, inhibition of IL11 signalling with X203 in TGFβ-stimulated HDFs markedly reduced cell proliferation and the expression of collagen I and periostin (Fig. 2D and E and Supplementary Fig. S3C–E, available at Rheumatology online).
ERK signalling is important for IL11 effects in cardiac fibroblasts [9] and we found that TGFβ2 or IL11 stimulated ERK phosphorylation to a similar degree in HDFs (Supplementary Fig. S3F, available at Rheumatology online). We tested the mechanistic importance of ERK signalling and found that pharmacological inhibition of ERK activity blocked the profibrotic effects of all TGFβ isoforms with the same effect as neutralizing IL11 antibody (Fig. 2D and E and Supplementary Fig. S3C-E, G, available at Rheumatology online).
Finally and importantly, the use of IL11 neutralizing antibodies in SSc fibroblasts was shown to recapitulate the results seen in non-diseased HDFs. At baseline, SSc fibroblasts highly express IL11RA (Supplementary Fig. S3H, available at Rheumatology online) and stimulation of SSc fibroblasts with IL11 or TGFβ2 induced αSMA expression (Fig. 2F and G), collagen I and periostin, and also increased cell proliferation (Supplementary Fig. S3I, available at Rheumatology online). Following TGFβ2 stimulation, inhibition of IL11 signalling with either X203 or ERK inhibition (U0126, 10 μM) resulted in a reduction in all profibrotic phenotypes tested (Supplementary Fig. S3I, available at Rheumatology online).
Discussion
While many factors stimulate HDF activation, members of the TGFβ superfamily are regarded as the prototypical activators. Using fibroblasts explanted from healthy controls and patients with diffuse SSc treated with TGFβ, Sargent et al. [15] identified IL6 and IL11, among others, as TGFβ-responsive genes in vitro. More recently, IL11 has been found to be highly upregulated in SSc skin fibroblasts [6] and in pulmonary fibroblasts of patients suffering from SSc-associated interstitial lung disease [11]. Of all the IL6 family cytokines, IL11 is the only member whose expression correlates with lung fibrosis severity in patients with idiopathic pulmonary fibrosis [10]. While targeting IL6 in SSc has proved unsuccessful for the primary end point of improvement in skin sclerosis (focuSSced trial, NCT02453256) [16], perhaps reflecting the low levels of IL6 receptor (IL6R) on fibroblasts [9], the role of IL11 has remained unexplored.
In this study, we show that patients with early diffuse SSc have elevated serum levels of IL11 and that HDFs highly express IL11RA but not IL6R. In keeping with data on fibroblasts from other organs [12], we show that IL11 is required for the profibrotic effects of TGFβ in HDFs and that this is primarily driven via non-canonical ERK signalling. This is in agreement with previous work describing the central role for the ERK1/2 cascade in relation to TGFβ-stimulated SSc fibroblast activation in vitro [17–19] and skin fibrosis in vivo [19]. We conclude that non-canonical TGFβ and IL11 signalling act through ERK to cause fibrosis and that the effect downstream of TGFβ is IL11-dependent.
Some studies have suggested a role for STAT3 in the profibrotic effects of TGFβ [20–22]. We found that IL11 consistently induced ERK phosphorylation concomitant with αSMA upregulation, while STAT3 phosphorylation was limited, not sustained and was only apparent at supraphysiological IL11 levels. Thus, we think it is unlikely that STAT3 activity is directly related to IL11 effects on fibrosis phenotypes although indirect mechanisms could play a role. Our data also rule out a dependency of IL11 on canonical TGFβ signalling as IL11-stimulated HDFs, while exhibiting pro-fibrotic phenotypes, do not upregulate TGFβ and show no evidence of SMAD2/3 activation. We point out that it is possible that IL11 induces reactive oxidative species in HDFs, given that IL11 promotes NADPH oxidase 4 (NOX4) upregulation in hepatocytes and NOX4 is strongly upregulated in the skin of patients with SSc [6, 23, 24], and this requires further study.
SSc remains a disease for which no treatment has proven uniformly effective. Unfortunately, anti-TGFβ interventions have generic and recurrent concerns over efficacy and safety [7, 8]. More recently, molecules targeting integrin-mediated TGFβ activation have been developed to attempt to bypass toxicities. However, TGFβ2 is the most dysregulated TGFβ isoform in SSc patients and this isoform is refractory to integrin-mediated activation. Thus the utility of integrin targeting therapies in SSc is open to question and other approaches may be needed.
In conclusion, we present evidence for the involvement of IL11, a TGFβ responsive and pro-fibrotic cytokine that is highly upregulated in SSc fibroblasts, in HDF activation. If IL11 is shown to promote skin fibrosis in in vivo disease models, IL11 may represent a new and accessible therapeutic target for dermal fibrosis in SSc and perhaps other conditions.
Supplementary Material
Acknowledgements
We would also like to thank the NHCS sequencing team for their technical expertise. S.A.C., E.A. and S.V. designed the study and planned experiments. S.V., E.A., A.A.W., B.N., J.T. and B.L.G. carried out molecular biology experiments; S.V., E.A., A.A.W. and B.N. analysed the data. L.P.M. prepared RNA-seq libraries and S.C. and N.Z. analysed sequencing data. Human samples acquisition and preparation: S.O.R., A.L.H.L., S.A., B.R., U.A., K.G. and B.S.P. E.A., A.A.W., S.S. and S.A.C. prepared the manuscript with input from co-authors.
Funding: This research is supported by the National Medical Research Council (NMRC), Singapore STaR awards (NMRC/STaR/0029/2017), NMRC Centre Grant to the NHCS, MOH‐CIRG18nov‐0002, Goh Foundation, Tanoto Foundation, NMRC CIRG14nov021. S.A. is supported by the NMRC (NMRC/STaR/020/2013, NMRC/MOHIAFCAT2/2/08, TCR15Jun006, NMRC/CIRG/1460/2016, and NMRC/CG/M003/2017), by Duke-NUS, BMRC (SPF2014/005), and by Duke-NUS and SingHealth core funding. A.A.W. is supported by NMRC/OFYIRG/0053/2017.
Disclosure statement: S.A.C., S.S., A.A.W. and B.N. are co-inventors on a number of patent applications relating to the role of IL11 in human diseases that include the published patents: WO2017103108, WO2017103108 A2, WO 2018/109174 A2, WO 2018/109170 A2. S.A.C. and S.S. are co-founders and shareholders of Enleofen Bio PTE Ltd, a company (which S.A.C. is a director of) that developed anti-IL11 therapeutics, which was acquired for further development by Boehringer Ingelheim.
Data availability statement
Data are available under GEO accession number (GSE162966).
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Allanore Y, Simms R, Distler O. et al. Systemic sclerosis. Nat Rev Dis Primers 2015;1:15002. [DOI] [PubMed] [Google Scholar]
- 2. Matucci-Cerinic M, Kahaleh B, Wigley FM.. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum 2013;65:1953–62. [DOI] [PubMed] [Google Scholar]
- 3. Worrell JC, O'Reilly S.. Bi-directional communication: conversations between fibroblasts and immune cells in systemic sclerosis. J Autoimmun 2020;113:102526. [DOI] [PubMed] [Google Scholar]
- 4. Zeisberg M, Kalluri R.. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 2013;304:C216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shin JY, Beckett JD, Bagirzadeh R. et al. Epigenetic activation and memory at a TGFB2 enhancer in systemic sclerosis. Sci Transl Med 2019;11:eaaw0790. doi: 10.1126/scitranslmed.aaw0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denton CP, Ong VH, Xu S. et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann Rheum Dis 2018;77:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denton CP, Merkel PA, Furst DE. et al. ; Cat-192 Study Group; Scleroderma Clinical Trials Consortium. Recombinant human anti-transforming growth factor β1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 2007;56:323–33. [DOI] [PubMed] [Google Scholar]
- 8. Varga J, Pasche B.. Transforming growth factor β as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 2009;5:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schafer S, Viswanathan S, Widjaja AA. et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature 2017;552:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng B, Dong J, D’Agostino G. et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med 2019;11:eaaw1237. [DOI] [PubMed] [Google Scholar]
- 11. Lindahl GE, Stock CJ, Shi-Wen X. et al. Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respir Res 2013;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook SA, Schafer S.. Hiding in plain sight: interleukin-11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu Rev Med 2020;71:263–76. [DOI] [PubMed] [Google Scholar]
- 13. Nieminen P, Morgan NV, Fenwick AL. et al. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am J Hum Genet 2011;89:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Widjaja AA, Singh BK, Adami E. et al. Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology 2019;157:777–92.e14. [DOI] [PubMed] [Google Scholar]
- 15. Sargent JL, Milano A, Bhattacharyya S. et al. A TGFβ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Invest Dermatol 2009;130:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khanna D, Lin CJF, Furst DE. et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2020;8:963–74. [DOI] [PubMed] [Google Scholar]
- 17. Akhmetshina A, Dees C, Pileckyte M. et al. Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum 2008;58:2553–64. [DOI] [PubMed] [Google Scholar]
- 18. Reich N, Maurer B, Akhmetshina A. et al. The transcription factor Fra-2 regulates the production of extracellular matrix in systemic sclerosis. Arthritis Rheum 2010;62:280–90. [DOI] [PubMed] [Google Scholar]
- 19. Gerber EE, Gallo EM, Fontana SC. et al. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 2013;503:126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chakraborty D, Šumová B, Mallano T. et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun 2017;8:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dees C, Tomcik M, Palumbo-Zerr K. et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor β in systemic sclerosis. Arthritis Rheum 2012;64:3006–15. [DOI] [PubMed] [Google Scholar]
- 22. O'Reilly S, Ciechomska M, Cant R, van Laar JM.. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J Biol Chem 2014;289:9952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piera-Velazquez S, Makul A, Jiménez SA.. Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor β. Arthritis Rheumatol 2015;67:2749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Widjaja AA, Dong J, Adami E, Viswanathan S, Ng B, Singh BK.. Redefining Interleukin 11 as a regeneration-limiting hepatotoxin. bioRxiv, doi: 10.1101/830018, 4 November 2019, preprint: not peer reviewed. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available under GEO accession number (GSE162966).