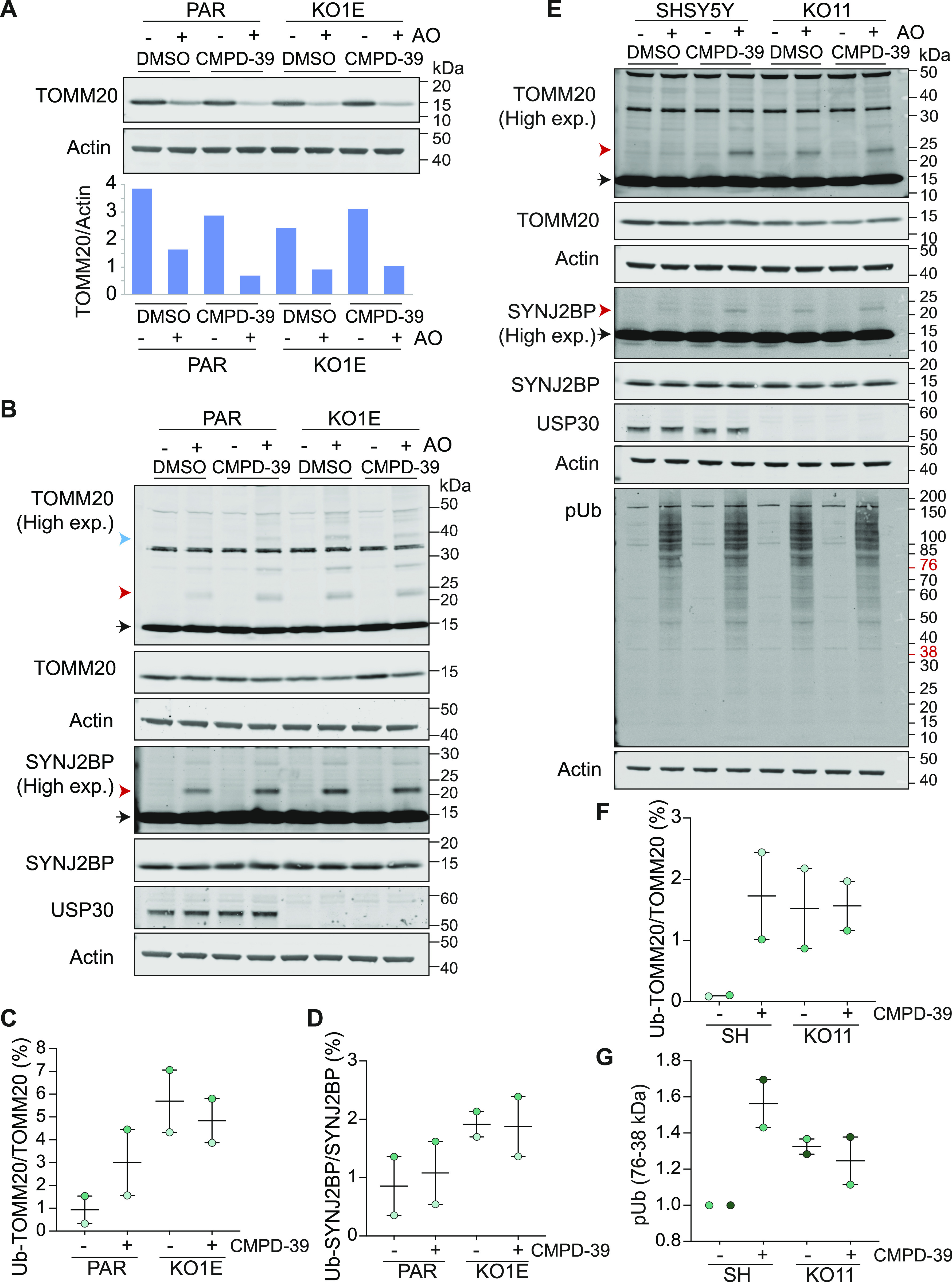
Figure 2. CMPD-39 promotes depolarization-dependent ubiquitylation of previously described USP30 substrates.
(A) USP30 inhibitor (CMPD-39) treatment of Parental (PAR) hTERT-RPE1-YFP-PRKN cells phenocopies USP30 deletion (KO1E) by promoting TOMM20 degradation, whereas TOMM20 degradation is unaffected by CMPD-39 in the USP30 KO (KO1E) cells. Cells were treated for 4 h ± AO (1 μM) ± 200 nM CMPD-39. (B) TOMM20 and SYNJ2BP ubiquitylation is enhanced within 1 h by CMPD-39 but is unaffected in the USP30 KO (KO1E) cells. Cells were treated for 1 h ± AO (1 μM) ± 200 nM CMPD-39. (C, D) Graphs show quantification of mono-ubiquitylated TOMM20 and SYNJ2BP in AO-treated samples as a percentage of total for two independent experiments (error bars indicate the range). (E) USP30 inhibitor (CMPD-39) treatment of cells expressing endogenous PRKN (SHSY5Y cells) similarly phenocopies USP30 deletion (KO11) by promoting TOMM20 and SYNJ2BP ubiquitylation, as well as increasing levels of phospho-Ser65 Ubiquitin (pUb). Cells were treated for 4 h ± AO (1 μM) in the absence or presence of 1 μM CMPD-39. (E, F, G) Graphs show quantification of AO treated samples for mono-ubiquitylated TOMM20 as a percentage of total TOMM20 (F) and the pUb signal normalised to AO treated SHSY5Y cells (G) in the 38–76 kD range, for two independent experiments represented by (E) (error bars indicate the range). (B, E): Black arrow indicates unmodified species, and red and blue arrow heads indicate the mono- and multi-ubiquitylated species, respectively.
Source data are available for this figure.