Abstract
Background
The overlap of malaria and Hepatitis B Virus (HBV) infections present a major threat to public health throughout endemic countries of tropical and sub-Saharan Africa. There is a paucity of data on the prevalence and associated factors of malaria and HBV infections among pregnant women in Ejule, a semiurban area of Nigeria. Therefore, the current study was designed to assess the seroprevalence of malaria and HBV among pregnant women attending antenatal clinics in Ejule Metropolis.
Materials and Methods
In a hospital-based cross-sectional study, blood samples collected from 200 apparently healthy pregnant women at the Ilemona Clinic were screened for Plasmodium falciparum (P. falciparum) and HBsAg using histidine-rich protein 2 (HRP2) and hepatitis B surface antigen (HBsAg) rapid diagnostic tests (RDTs), respectively. Relevant sociodemographic and putative risk factor information was obtained with structured questionnaires.
Results
The prevalence of the infections was 44 (22%), 5 (2.5%), and 1 (0.5%) for P. falciparum monoinfection and HBV monoinfection and coinfection, respectively. Single and concurrent infections peaked at ages 31–40 years but decreased with older ages. High P. falciparum, 31 (59.62%), and HBV 2 (3.85%) infection were observed among those without formal education. Contrary to ages, occupation, and knowledge of infection, malaria parasitemia differed significantly with lower educational qualification (p ≤ 0.001), being single (p=0.001), and inconsistent use of insecticide-treated bed nets (ITNs) (p=0.04, OR = 5, CI: 0.10–0.47). History of blood donation (OR = 5, p=0.04, CI: 1.10–32.80) and multiple sex partners (OR = 11.9, p=0.01, CI: 0.01–0.93) were found to be significantly associated with hepatitis B surface antigenemia rate during pregnancy. No evidence of HBV infection was observed in women with a history of HBV vaccination.
Conclusions
Malaria is still highly prevalent among pregnant women due to high illiteracy and noncompliance to using ITNs. Therefore, routine screening and educating pregnant mothers are crucial in eliminating malaria in endemic settings. The low rate of hepatitis B and coinfection with malaria shows that further improvement in HBV vaccination could considerably reduce the disease burden among pregnant women.
1. Introduction
Malaria remains a disease of major public health concern globally. A world health report in 2020 showed malaria was responsible for about 229 million cases and 409 000 deaths worldwide [1]. Countries in sub-Saharan Africa and India with 85% of the global malaria burden bear the brunt of the disease. Globally, Nigeria has 27% of the cases and is ranked first among the six countries that accounted for more than half of all malaria cases followed by the Democratic Republic of the Congo (12%), Uganda (5%), Mozambique (4%), and Côte d'Ivoire, Angola, and Niger (3% each) [1]. In areas where malaria is endemic, pregnant women with lowered immunity are particularly at risk for malaria infection and to develop complications of the disease that results in mortality [2]. Factors such as the increased body surface and specific odor secretions during pregnancy, which may attract more mosquito bites, have been linked to a higher incidence of malaria during pregnancy than their absence [2, 3].
Hepatitis B virus (HBV) is the leading cause of preventable liver disease and death globally [4, 5]. HBV was responsible for approximately 257 million carrier cases, including active and inactive chronic forms with a global annual death rate estimated at 887 000 [4]. In Nigeria, HBV had a national prevalence of 11% in 2018, and according to the Ministry of Health, infection with the virus has become the leading silent killer due to nondetection in more than half of the nation's population who have never been tested and are not showing symptoms [6]. In 2020, the world health report showed about 20 million Nigerian population were living with HBV [7]. Viral transmission in the country is potentiated through exposure to infectious blood or blood products or by means of percutaneous exposure to sharp contamination [5].
Malaria and HBV coinfection may pose a serious public health problem among pregnant women in developing countries where both infectious diseases are endemic [8]. Despite having different transmission routes, the overlapping endemicity or geographical coincidence of both infections predispose people who reside in the endemic territory to coinfection [8]. A study by Scotto and Fazio [9] posited that since HBV is highly contagious from one infected individual to another, especially in the tropics, chances of viral transmission through the same route of transmission of Plasmodium vis-à-vis blood-to-blood contact, sharing needles, and blood transfusions are high. Asymptomatic malaria parasitic infection and full-blown illness with typical febrile paroxysms are the heights of the wide spectrum of clinical diseases caused by malaria parasites. People who live in malaria-endemic areas have a greater chance of developing asymptomatic malaria due to their acquired semi-immunity with submicroscopic levels of parasitemia. Coinfection and pregnancy-induced immunosuppression may likely modulate the clinical progression and severity of malaria [9]. Both malaria and HBV infections share some of their developmental stages within the hepatocytes, and such co-occurrence has been attributed to increased liver injury associated with synergistic multiplication of P. falciparum [10] and rapid progression of HBV infection, as well as the natural history of both diseases [11]. The mutual effect of coinfection in pregnancy aggravates clinical manifestations of malaria, which can culminate in poor pregnancy outcomes such as low birth weight, intrauterine growth restriction, maternal anemia and fetal mortality [12]. In Kogi State, reports of HBV or malaria monoinfection have been documented [13–15]. However, little or no information exists with regards to HBV and malaria coinfection in the north-central region of Nigeria, albeit in pregnant women seeking antenatal care in Ejule, Nigeria. The current study aimed to evaluate seropositivity to HBsAg and malaria parasite antigen singly and concurrently among pregnant women attending the antenatal clinic in Ejule metropolis, Kogi State, Nigeria.
2. Materials and Methods
2.1. Study Population/Design
The study was carried out at Ejule, which is situated in the eastern part of Kogi State found in the north-central region of Nigeria. Ejule town has a projected population of 24,831 people in 2020 from the 2006 population census (Center for International Earth Science Information Network, https://www.city-facts.com/ejule). Indigenes are predominantly Igala with agriculture as a major occupation. A total of 200 randomly selected apparently healthy consenting antenatal attendees aged 15–51 years were recruited at Ilemona Clinic in Ejule from September to November 2019. The hospital runs weekly services for antenatal women and is the reference hospital in the area. The number of visits to Ilemona Clinic is estimated at 180 patients/week. In this cross-sectional study, all subjects signed an informed consent form before being enrolled in the study and agreed with the publication of the findings related to the study. For participants younger than 18 years of age, their parents or guardian signed the consent form. Ethics approval for the study was obtained from the hospital management board on health issues after the due process was followed (Ethical clearance no: 058).
2.2. Specimen Collection and Preparation
Four (4) mL of blood was aseptically collected from each of the 200 consenting subjects by the venepuncture method into a well-labeled anticoagulant tube. The samples were transported to the microbiology department under cold conditions. The blood was spun at 3000 rpm for 8–10 minutes to separate sera from whole blood. Sera were stored at −20°C until screened for HBsAg and malaria parasite antigen. Relevant demographic variables (e.g., level of education, occupation, and marital status) and putative risk factor information (e.g., history of blood transfusion, alcoholism, mosquito net use, and multiple sexual partners) were obtained as appropriate with a closed-ended structured questionnaire with the assistance of a local language translator.
2.3. Laboratory Assays for HBsAg and Malaria Parasite
The commercial HBsAg test kit (ABON Biopharm Inc., Ltd., Hangzhou, China) was employed for the qualitative detection of hepatitis B surface antigen in serum. The rapid diagnostic test (RDT) kit (Standard Diagnostics, Inc., Korea) for P. falciparum was used to test for malaria parasites in blood samples. The RDT kit for P. falciparum was used since the species is the most predominant in Nigeria [1]. Analysis was carried out in the microbiology laboratory, Kogi State University, Anyigba. All test procedures and interpretation of the results were performed in accordance with the protocol of the kit's manufacturers.
2.4. Statistical Analysis
Statistical Packages for Social Sciences (SPSS) version 16 for windows (SPSS Inc. Chicago, IL) was used to analyze data obtained in the study. Chi-square statistics was used to compare the difference between proportions. Logistic regression analysis at a 95% confidence interval was used to determine odds ratios used to measure putative risk factors of HBV and malaria transmission. A p value of ≤0.05 was considered statistically significant.
3. Results
Of the 200 study participants screened for malaria parasite and HBsAg, 44 (22%) were positive for malaria and 5 (2.5%) for HBsAg, while 1 (0.5%) sample was positive for both malaria parasite and HBsAg (Table 1). The age group 31–40 years had a higher prevalence of either HBV (3.33%), malaria (25.0%), or both infections (0.83%) than the other age groups 15–20 years with 0%, 5.88%, and 0%; 21–30 years with 2.27%, 20.45%, and 0%; 41–50 years with 0%, 21.43%, and 0%; and >50 years with 0%, 20%, and 0% for HBV, malaria, and coinfections, respectively. The presence of a malarial infection is associated with 0.9 odds (1 × 157/4 × 43 = 0.9) of being coinfected with HBV in the study. Generally, infection with HBV or malaria increased progressively from 15–20 years, peaked at ages 31–40 years, and then, decreased afterward with advancing age. Despite the increasing trend of infection with ageing, the study observed no significant difference among age groups of exposure to each infection and the prevalence rates (p > 0.05). In comparison with the other occupational status, women who practiced farming had a higher prevalence of malaria (25.76%) and HBV (3.03%) infection followed by housewives with HBsAg and malaria parasite positivity of 2.94% and 22.55%, respectively. A dual infection (0.5%) was observed in a woman practicing farming. A statistically significant difference was observed between the occupation of subjects and malaria/HBV coinfection (p=0.03). There were more malaria, 31 (59.62%), and HBV 2 (3.85%) infections among women who lacked formal education. Except HBV and HBV/malaria coinfection (p=0.81, 0.41), the prevalence of malaria alone differed significantly with the educational level of subjects (p ≤ 0.001). Generally, a decreasing trend in HBsAg and/or malaria parasitemia was observed as the level of education increases. More single women (42.86%) were infected with malaria than married (17.56%) and divorced (0.0%) women. There was a statistically significant difference between marital status and malaria infection (p=0.001). Similarly, more single women (8.57%) were infected with HBV than married (1.21%) and divorced (0%) women, and the difference between marital status and hepatitis B surface antigenemia rates was significant (p=0.03) (Figure 1). Previous exposure to donated blood was significantly linked to higher HBsAg seropositivity as women with such history had 5 times as many chances of contracting HBV infection than those without such history (OR = 5, p=0.04, CI: 1.10–32.80). Knowledge of HBV infection prevention tends to play a role in the study as about 3% of the seropositive subjects were those who never had prior knowledge of viral infection. Subjects with multiple sex partners had about 12 times more chances of being infected with HBV compared to those with one or two partners (OR = 11.9, p=0.01, CI: 0.01–0.93). The seropositivity rates to HBV were related to subjects' immunization status as 5 (2.75%) and zero prevalence rates among nonimmunized and immunized subjects, respectively, were observed in the current study (Table 2). A greater predisposition to malaria was observed among subjects who did not use mosquito net (34%) than those who use bed net consistently (10%). Seroprevalence differed significantly between consistent mosquito net users and nonusers (p=0.04, odds of not using mosquito net = 1/0.2 = 5, CI: 0.10–0.47) (Table 3).
Table 1.
The prevalence of HBV, malaria, and coinfection in relation to ages of pregnant women in Ejule.
| Age group (years) | No. tested | HBsAg +ve no. (%) | p value | Malaria +ve no. (%) | p value | Coinfection no. (%) | p value |
|---|---|---|---|---|---|---|---|
| 15–20 | 17 | 0 (0) | 1 (5.88) | 0 (0) | |||
| 21–30 | 44 | 1 (2.27) | 9 (20.45) | 0 (0) | |||
| 31–40 | 120 | 4 (3.33) | 0.086 | 30 (25.0) | 0.51 | 1 (0.83) | 0.96 |
| 41–50 | 14 | 0 (0) | 3 (21.43) | 0 (0) | |||
| 51 above | 5 | 0 (0) | 1 (20) | 0 (0) | |||
| Total | 200 | 5 (2.5) | 44 (22.0) | 1 (0.5) |
Figure 1.
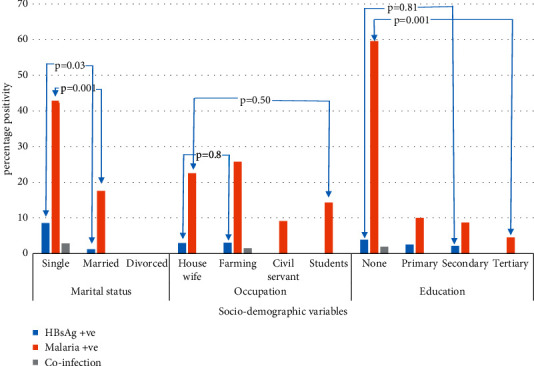
Prevalence of HBV, malaria, and coinfection in relation to socialdemographic factors.
Table 2.
Logistic regression analysis of HBV infection in relation to putative risk factors.
| Variable | HBsAg +ve no. (%) | OR (95% CI) | p value |
|---|---|---|---|
| History of blood transfusion | |||
| Yes (n = 46) | 3 (6.52) | ||
| No (n = 154) | 2 (1.30) | 5.3 (1.10–32.80) | 0.04 |
| Knowledge of HBV | |||
| Yes (n = 20) | 0 (0) | ||
| No (n = 180) | 5 (2.78) | — | 0.22 |
| Alcoholism | |||
| Yes (n = 42) | 1 (2.38) | ||
| No (n = 158) | 4 (2.53) | 0.9 (0.12–9.79) | 0.96 |
| Multiple sex partners | |||
| Yes (n = 5) | 1 (20) | ||
| No (n = 195) | 4 (2.05) | 11.9 (0.01–0.93) | 0.01 |
| Sharing of sharp objects | |||
| Yes (n = 120) | 3 (2.5) | ||
| No (n = 80) | 2 (2.5) | 1 (0.16–6.12) | 1.00 |
| Tribal mark | |||
| Yes (n = 104) | 2 (1.92) | ||
| No (n = 96) | 3 (3.13) | 0.6 (0.27–10.06) | 0.59 |
| Immunized | |||
| Yes (n = 18) | 0 (0) | ||
| No (n = 182) | 5 (2.75) | — | 0.48 |
| Intravenous drug use | |||
| Yes (n = 15) | 0 (0) | ||
| No (n = 185) | 5 (2.70) | — | 0.52 |
| Surgery | |||
| Yes (n = 35) | 1 (2.86) | ||
| No (n = 165) | 4 (2.42) | 1.2 (0.13–10.90) | 0.88 |
CI = confidence interval; OR = odds ratio.
Table 3.
Logistic regression analysis of malaria parasite infection in relation to putative risk factors.
| Variables | No. tested | Malaria +ve no. (%) | OR (95% CI) | p value |
|---|---|---|---|---|
| Use of mosquito treated net | ||||
| Yes | 100 | 10 (10) | 0.2 (0.10–0.47) | 0.04 |
| No | 100 | 34 (34) | ||
| Knowledge of malaria | ||||
| Yes | 45 | 9 (20) | 0.9 (0.38–1.95) | 0.79 |
| No | 155 | 35 (22.58) |
4. Discussion
Malaria and hepatitis B virus infections are both common and life-threatening infectious diseases in developing countries including Nigeria, and the endemicity of these infections overlaps frequently due to geographical coincidence. Their high prevalence as monoinfection shows their infectious ability as coinfections. In a coinfected individual, both infections can influence each other from a clinical perspective because of some common developmental stages within the hepatocytes, which had been likened to impaired clearance of the liver stages of malaria parasites due to hepatocytes damage in hepatitis B virus infection [9, 16].
In the current study, the prevalence of malaria, hepatitis B, and coinfections was 22.0%, 2.5%, and 0.5%, respectively. The malaria prevalence in our study is comparable with the national prevalence rate of 25% [1], 22.4% in Cameroon [17], 20.4% in Ghana [18], and 28.8% earlier reported among pregnant women from other parts of the state [13]. Lower prevalence rates of 10.2%, 11.6, and 7.7% in Ethiopia, Ghana, and Nigeria, respectively, have been reported [19–21]. The plausible reason for these variations could be attributed to the marked seasonal, interannual, and spatial variability among the study areas. For instance, the current study was carried out during the rainy season with a long stretch of flooding, which could constitute suitable breeding sites for malaria vectors, causing increased vector-human interactions and malaria transmission. Contrary to our findings, relatively higher malaria parasitemia rates have been reported in other studies, in Nigeria (58%) and Ghana (42%), respectively [22, 23]. The differences in sociodemographic variables, transmission dynamics, malaria prevention measures, laboratory diagnostic tool used, adherence to intermittent preventive treatment, general health awareness, and levels of immunity in different locations are possible contributing factors to the prevalence of P. falciparum infections observed in the abovementioned literature.
The high rate of P. falciparum monoinfection among the less-educated women might be due to their poor lifestyle orchestrated by their inability to take adequate measures in infection control and prevention. These findings corroborate the previous report of Anabire et al. [24] in Northern Ghana. Thus, a rigorous health education program targeted at the less-enlightened women that can address the social, occupational, cultural, and economic factors associated with increased predisposition to infection could potentially control the comorbidity in most of the susceptible and underserved groups in this region and the country at large.
The rationale for the high malaria infection among women whose occupation was farming could be due to their poor awareness about infection control. Our findings are supported by the assertion of Okolo et al. [14] and Omatola et al. [15] who posited that these occupational groups, which are usually less enlightened, often overlooked the need to be properly clothed against mosquito bites during farming activities, fail to use mosquito nets consistently, and still believe in traditional rituals such as tribal marks/scarification or use of unscreened blood for transfusion that could expose them to infection.
The P. falciparum/HBV coinfection rate of 0.5% in the current study is lower than the prevalence rates of 4.3%, 6%, and 0.7% earlier reported in parts of Nigeria [12], Gambia [8], and Ghana [20], respectively. None of the subjects with a history of vaccination show any evidence of HBV infection, suggesting that immunity provided through HBV vaccination in the studied population could have contributed to the low HBV monoinfection rate and by implication lower comorbid state. The ability of the HBV vaccine to offer 98–100% protection against hepatitis B infection has been documented [7]. The hepatitis B surface antigenemia and its co-occurrence rate with malaria parasitemia, although low in the current study, are detrimental to the life of pregnant women and the yet unborn child. The fact that both HBV and Plasmodium falciparum share their life cycle in the liver suggests they could significantly influence the health and well-being of pregnant women and their fetuses in events of coinfections as has been previously reported [20]. Most babies delivered with mothers who are chronic carriers of HBV usually become chronically infected at birth if there is no prevention [25].
The high prevalence of P. falciparum monoinfection among women aged 31–40 years might be explained by their higher-risk behaviors for infection by both Plasmodium spp. and HBV, including sexual promiscuity, fashionable tattooing, and the lack of consistent mosquito nets used for beds than other age groups [12]. These ages coincide with the peak age for Plasmodium spp. and HBV coinfection suggested in a meta-analysis study of Kotepui and Kotepui [8]. The decline in mono- or coinfection with advancing age might be attributed to immunity, which is normally acquired against malaria parasites [14] or decline in the tendency for sexual promiscuity and other risky behaviors to contracting HBV infection [15]. Another possible reason is that mothers with increased age are more likely than the younger ones to have better exposure to health services and greater awareness about malaria and or hepatitis B and the ways of prevention through antenatal health talks.
We observed significant P. falciparum and HBV monoinfection among single women in the study area. These could be attributed to the absence of family cover, which can increase the tendency for outdoor adventures (e.g., outdoor sleeping and camping) that predisposed people to mosquito bites or unrestricted sexual activities without protection [15]. Women with multiple sex partners in the study had about 12 times odds of being monoinfected with HBV. Also, subjects with a history of blood transfusion were 5-fold more likely to be monoinfected with HBV. This finding supports the assertion of Scotto and Fazio [9] who posited that since HBV is highly contagious in the tropics, chances of viral transmission through the same route of transmission of Plasmodium vis-à-vis blood-to-blood contact, sharing needles, and blood transfusions are not uncommon in the endemic region. The high HBV and malaria antigenemia among women with poor knowledge of HBV or malaria infection prevention in the current study has been documented in the area [26]. Consequently, there is the need to intensify health education of the general population of Ejule on infection prevention and control strategies. Additionally, we found nonusers or inconsistent users of the insecticide-treated net with higher odds of P. falciparum monoinfection than consistent users, which has been reported in most studies [19, 27]. This finding might be explained by the fact that insecticide-treated nets are available to only the informed Nigerian population at a cost. Therefore, there is the need for government to make insecticide-treated mosquito nets available freely or at a subsidized rate to pregnant women and the masses to reduce both the number of malaria cases and associated sequelae.
One limitation of the study is that PCR, which is a more sensitive means of identification and confirmation of P. falciparum infection and occult HBV DNA, was not performed because of financial constraints. The use of less-sensitive methods suggests a higher problem on the ground and more sensitive PCR diagnostic methods should be used. Notwithstanding, the current study provides useful baseline epidemiological information regarding P. falciparum monoinfection and coinfection with HBV that future studies can build on in the study area. Our findings suggest the need for the initiation of routine screening of pregnant women for both hepatitis B and malaria at every antenatal care visit, as this would ensure timely management of cases and prevention of any possible adverse effects that could be associated with P. falciparum/HBV coinfection.
5. Conclusions
The high prevalence of malaria in the study area reaffirms its endemicity, which has been reported for most published studies in Nigeria. Low HBV monoinfection and coinfection with P. falciparum were observed. Women with the previous receipt of HBV vaccines show relative resistance to the viral infection. To considerably and rapidly reduce the burden of malaria and further decrease coinfection with hepatitis B, there is the need for government to strengthen malaria and hepatitis B prevention programs in all socioeconomic facets of the pregnant women population.
Acknowledgments
The authors express their profound gratitude to the staff and management of the Ilemona Clinic for their cooperation during the study. The authors also thank the pregnant women who participated in the study for showing interest and patience during the study.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
OAC and OMO designed the study, obtained ethical approval, and wrote the draft manuscript; OMO ran the assay; and OAC analyzed the data. All authors read and approved the final manuscript.
References
- 1.World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges . Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 2.Tegegne Y., Asmelash D., Ambachew S., Eshetie S., Addisu A., Jejaw Zeleke A. The prevalence of malaria among pregnant women in Ethiopia: a systematic review and meta-analysis. Journal of Parasitology Research . 2019;2019:9. doi: 10.1155/2019/8396091.8396091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yartey J. E. Malaria in pregnancy: access to effective interventions in Africa. International Journal of Gynecology & Obstetrics . 2006;94(3):364–373. doi: 10.1016/j.ijgo.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 4. WHO, Factsheet on Hepatitis B, 2020, https://www.who.int/news-room/fact sheets/detail/hepatitis-b.
- 5.Omatola C. A., Onoja B. A., Agama J. Detection of hepatitis B surface antigen among febrile patients in ankpa, Kogi state, Nigeria. Journal of Tropical Medicine . 2020;2020:6. doi: 10.1155/2020/5136785.5136785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abutu A. Nigeria’s complicated hepatitis burden. The Lancet Gastroenterology & Hepatology . 2018;3(10):p. 669. doi: 10.1016/s2468-1253(18)30279-6. [DOI] [Google Scholar]
- 7. WHO, World Hepatitis Day-In Nigeria, 2020, https://www.afro.who.int/news/world-hepatitis-day-nigeria-estimated-20-million-people-are-chronically-infected.
- 8.Kotepui K. U., Kotepui M. Prevalence of and risk factors for Plasmodium spp. co-infection with hepatitis B virus: a systematic review and meta-analysis. Malaria Journal . 2020;19(368):368–417. doi: 10.1186/s12936-020-03428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scotto G., Fazio V. Hepatitis B and asymptomatic malaria coinfection in Sub-Saharan African immigrants: epidemiological and clinical features of HBV infection. Revista da Sociedade Brasileira de Medicina Tropical . 2018;51(5):578–583. doi: 10.1590/0037-8682-0430-2017. [DOI] [PubMed] [Google Scholar]
- 10.Barcus M. J., Hien T. T., Laras K., et al. Short report: hepatitis B infection and severe Plasmodium falciparum malaria in Vietnamese adults. The American Journal of Tropical Medicine and Hygiene . 2002;66(2):140–142. doi: 10.4269/ajtmh.2002.66.140. [DOI] [PubMed] [Google Scholar]
- 11.Braga W. S. M., Silva E. B. d., Souza R. A. B. d., Tosta C. E. Soroprevalência da infecção pelo vírus da hepatite B e pelo plasmódio em Lábrea, Amazonas: estimativa da ocorrência de prováveis coinfecções. Revista da Sociedade Brasileira de Medicina Tropical . 2005;38(3):218–223. doi: 10.1590/s0037-86822005000300002. [DOI] [PubMed] [Google Scholar]
- 12.Abah A. E. U. I. Co-infection of malaria and hepatitis B virus in port harcourt, rivers state Nigeria. International Journal of Infection . 2019;6 doi: 10.5812/iji.97033.e97033 [DOI] [Google Scholar]
- 13.Mofolorunsho C. K., Audu H. O., Omatola C. A. Prevalence of malaria among pregnant women attending a healthcare facility in lokoja, North-central, Nigeria. Asian Journal of Pharmaceutical and Health Sciences . 2014;4(2):936–939. [Google Scholar]
- 14.Okolo M. O., Omatola C. A., Ezugwu A. I., Adejo P. O., Abraham O. J., Chukwuma O. J. T. Prevalence of malaria among pregnant women attending antenatal clinic in grimard catholic hospital, Anyigba in Kogi state, Nigeria. Nature and Science . 2017;15(9):113–117. [Google Scholar]
- 15.Omatola C. A., Idofe J., Okolo M.-L. O., Adejo P. O., Maina M. M., Oyiguh J. A. Seroprevalence of HBV among people living with HIV in Anyigba, Kogi state, Nigeria. African Health Sciences . 2019;19(2):1938–1946. doi: 10.4314/ahs.v19i2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrade B. B., Santos C. J. N., Camargo L. M., et al. Hepatitis B infection is associated with asymptomatic malaria in the Brazilian amazon. PLoS One . 2011;6(5) doi: 10.1371/journal.pone.0019841.e19841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anchang-Kimbi J. K., Nkweti V. N., Ntonifor H. N., et al. Plasmodium falciparum parasitaemia and malaria among pregnant women at first clinic visit in the mount Cameroon area. BMC Infectious Diseases . 2015;15(1):p. 439. doi: 10.1186/s12879-015-1211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dosoo D. K., Chandramohan D., Atibilla D., et al. Epidemiology of malaria among pregnant women during their first antenatal clinic visit in the middle belt of Ghana: a cross sectional study. Malaria Journal . 2020;19(381):381–412. doi: 10.1186/s12936-020-03457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gontie G. B., Wolde H. F., Baraki A. G. Prevalence and associated factors of malaria among pregnant women in Sherkole district, Benishangul Gumuz regional state, West Ethiopia. BMC Infectious Diseases . 2020;20(573):573–578. doi: 10.1186/s12879-020-05289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helegbe G. K., Aryee P. A., Mohammed B. S., et al. Seroprevalence of malaria and hepatitis B Co-infection among pregnant women in tamale metropolis of Ghana: a cross-sectional study. The Canadian Journal of Infectious Diseases & Medical Microbiology . 2018;2018:12. doi: 10.1155/2018/5610981.5610981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agomo C. O., Oyibo W. A., Anorlu R. I., Agomo P. U. Prevalence of malaria in pregnant women in Lagos, south-West Nigeria. Korean Journal of Parasitology . 2009;47(2):p. 179. doi: 10.3347/kjp.2009.47.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ifeanyi U., Onyebuchi V., Ukamaka T., Ugochukwu B. Asymptomatic Plasmodium parasitaemia in pregnant Nigerian women: almost a decade after roll Back malaria. Malaria Journal . 2009;24:16–20. doi: 10.1016/j.trstmh.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Berry I., Walker P., Tagbor H., et al. Seasonal dynamics of malaria in pregnancy in West Africa: evidence for carriage of infections acquired before pregnancy until first contact with antenatal care. The American Journal of Tropical Medicine and Hygiene . 2018;98(2):534–542. doi: 10.4269/ajtmh.17-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anabire N. G., Aryee P. A., Abdul-Karim A., Quaye O., Awandare G. A., Helegbe G. K. Impact of malaria and hepatitis B co-infection on clinical and cytokine profiles among pregnant women. PLoS One . 2019;14(4) doi: 10.1371/journal.pone.0215550.e0215550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinbaum C. M., Mast E. E., Ward J. W. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology . 2009;49:S35–S44. doi: 10.1002/hep.22882. [DOI] [PubMed] [Google Scholar]
- 26.Oseni Okolo M.-L., Omatola C. A. Hepatitis B and syphilis prevalence and risk factors of transmission among febrile patients in a primary health facility in Kogi State, Nigeria. Journal of Immunoassay and Immunochemistry . 2021;42:1–10. doi: 10.1080/15321819.2021.1938607. [DOI] [PubMed] [Google Scholar]
- 27.Feleke D. G., Adamu A., Gebreweld A., Tesfaye M., Demisiss W., Molla G. Asymptomatic malaria infection among pregnant women attending antenatal care in malaria endemic areas of North-Shoa, Ethiopia: a cross-sectional study. Malaria Journal . 2020;19:67–76. doi: 10.1186/s12936-020-3152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


