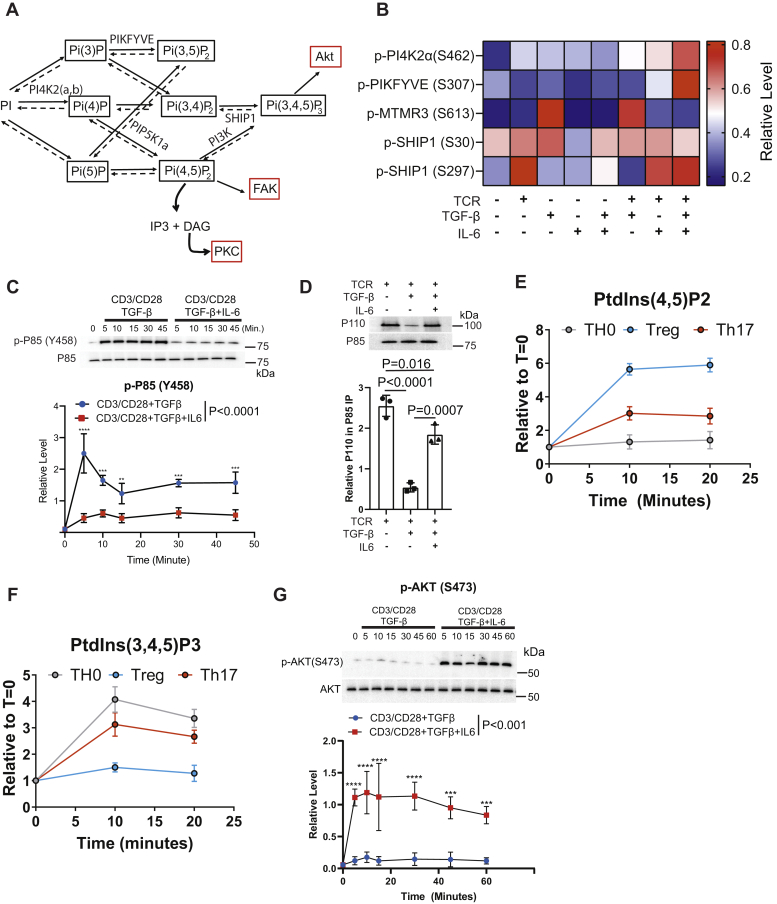
Figure 3.
Phosphatidylinositol metabolism is differentially regulated during Treg versus Th17 induction.A, a schematic of phosphatidylinositol metabolism is depicted. B, the label-free phosphoproteomic analysis determined the relative abundance of phosphopeptides for phosphatidylinositol kinases and phosphatases regulated by signaling input. C, primary CD4+ murine T cells isolated by negative selection were activated under Treg (anti-CD3 AB, soluble CD28 AB, and TGF-β) or Th17 (anti-CD3 AB, soluble anti-CD28 AB, TGF-β, and IL-6) induction conditions. Immunoblotting was performed for p-P85 (Y458) and total P85. Densitometry was performed across three biological replicates to quantitate p-P85 (Y458) normalized to total p85 levels. Shown are mean ± SD; p values were calculated by two-way ANOVA (∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01). D, primary murine CD4+ T cells were activated with under TH0 (anti-CD3, soluble anti-CD28 antibodies), Treg (anti-CD3 and soluble anti-CD28 antibody and TGF-β) and Th17 (anti-CD3 antibody, anti-CD28 antibody, TGF-β, and IL-6) for 10 min. P85 was immunoprecipitated. Immunoblotting was performed for P85 and P110. Densitometry was performed across three biological replicates. Each data point represents the mean ± standard deviation across three independent experiments and one-way ANOVA was performed. The abundances of (E) PtdIns(4,5)P2 and (F) PtdIns(3,4,5)P3 were measured with an imaging flow cytometry assay in primary murine CD4+ T cells activated under TH0, Treg, and Th17 conditions (defined above). Three biological replicates were performed. Presented is the mean ± standard deviation. G, primary murine CD4+ T cells were activated under Treg and Th17 conditions (defined above), and immunoblotting was performed on the resulting lysates for p-AKT(S473) and total AKT. Densitometry was performed on the immunoblots. Three biological replicates were included. Phosphorylated AKT was normalized to the total AKT abundance. Shown are mean ± SD; p values were calculated by two-way ANOVA (∗∗∗∗p < 0.0001, ∗∗∗p < 0.001). Source data are provided for the immunoblots in panels C, D, and G.