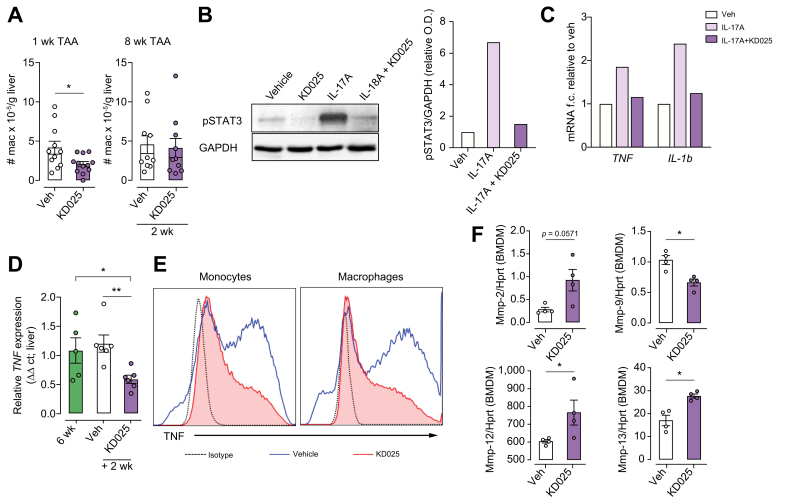
Fig. 5.
KDO25 alters macrophage function.
(A) C57BL/6 mice were treated for 1 week with TAA and vehicle or with TAA and KD025 (left) or were treated with TAA for 6 weeks followed by 2 weeks of TAA co-administration with vehicle or KD025 (right). The number of liver macrophages were quantified (n = 10−12 animals/group; combined from 2 independent experiments). (B) BMDM were treated with DMSO (vehicle), KD025 (10 μM), IL-17A (10 μg/ml), or KD025 + IL-17A for 20 min. Lysates were analysed by immunoblot using antibodies specific for pSTAT3 and GAPDH, with densitometric analyses shown. (C) BMDM were treated with DMSO (vehicle), IL-17A (10 μg/ml), or KD025 (10 μM) + IL-17A for 6 h. qRT-PCR analysis of TNF and IL-1β mRNA normalised to HPRT mRNA is shown. Data shown in (B) and (C) are representative of 2 independent experiments (n = 1/condition). (D) Mice were treated with TAA for 6 weeks or treated with TAA for 6 weeks followed by 2 weeks of co-administration with vehicle or KD025. qRT-PCR analysis of TNF mRNA expression in whole liver (n = 5–6 animals/group) is shown. (E) Isolated hepatic leucocytes from mice treated as in (D) were treated in vitro with LPS (100 ng/ml) and analysed by flow cytometry for monocyte (left) and macrophage (right) TNF production. (F) BMDM were treated with KD025 (10 μM) or DMSO control for 6 h, and the expression of mRNAs encoding MMPs, normalised to HPRT mRNA, was determined by qRT-PCR (n = 4 animals/group). Data are presented as mean ± SEM. ∗p <0.05, ∗∗p <0.01 Mann–Whitney U test. BMDM, bone marrow-derived macrophages; MMP, matrix metalloproteinase; O.D., optical density; pSTAT3, signal transducer and activator of transcription 3 phosphorylation; qRT-PCR, quantitative real-time PCR; TAA, thioacetamide.