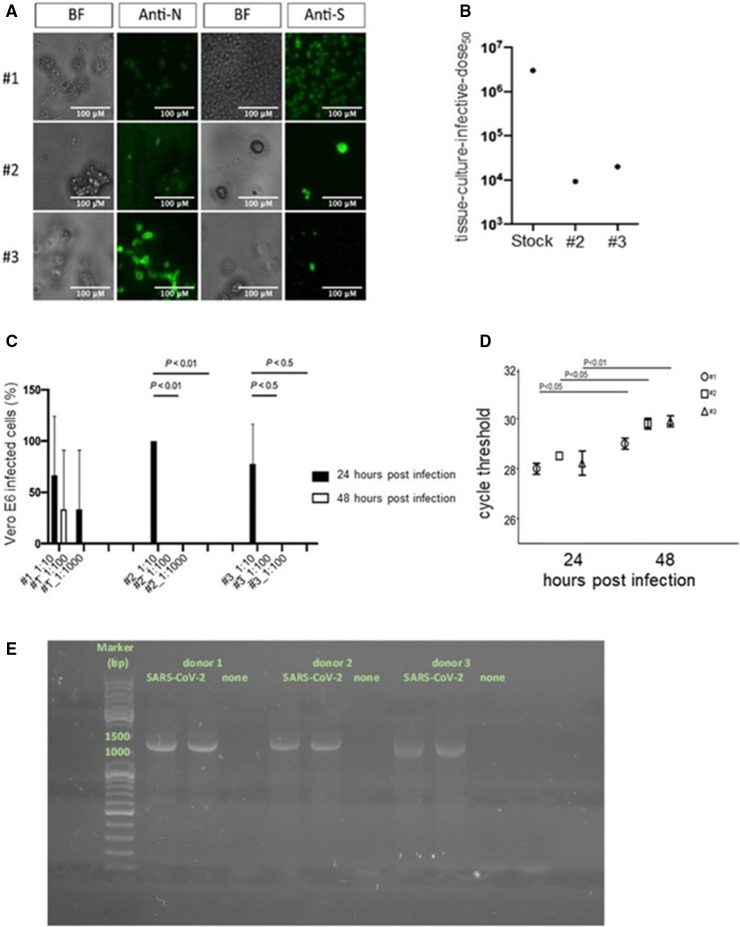
FIGURE 5.
SARS‐CoV‐2 interacts with but does not productively infect platelets. Bright‐field (BF) microscopy and immunofluorescence of platelets 48 h after challenge with a SARS‐CoV‐2 clinical isolate (SARS‐CoV‐2) stained with anti‐SARS‐CoV‐2 Nucleoprotein Abs (Anti‐N) or anti‐SARS‐CoV‐2 Spike Abs (Anti‐S). 20× magnification, scale bar 100 µm (A). Back‐titration performed on the unbound virus from donors 2 and 3, compared with viral stock used for the infection protocol (B). Cytopathic effect was assessed on Vero E6 cells using a scoring system to evaluate infectivity of platelets supernatants collected at 24 and 48 h postchallenge. Mean values for all experimental replicates and SD are reported with error bars (C). Quantitative RT‐PCR on supernatants collected from platelets challenged with the virus of the three donors, at 24 and 48 h. Cycle threshold levels, represented on the y axis, are inversely proportional to the amount of target nucleic acid in the sample (the lower the cycle threshold level the greater the amount of virus within the supernatant). Mean values for all experimental replicates, tested each one in duplicate in quantitative RT‐PCR, and SD are reported with error bars (D). Agarose gel electrophoresis PCR products of platelets treated with the clarified supernatants of infected (SARS‐CoV‐2) or uninfected Vero E6 cells (none) (E). [Correction added on November 25, 2021, after first online publication: Figure 5 has been updated in this version.]