Abstract
Pentacyclic Phytomolecule 3-O-Acetyl-11-keto-β-boswellic acid (AKBA) from Frankincense family has proven for the neuroprotection and recognized as an orphan drug for the treatment of cerebral edema. Nonetheless, AKBA have promising indications with Peroxisome proliferator activated receptor gamma (PPARγ) associated to cognitive function not deliberated so far. In order to substantiate the potential role of AKBA on memory function, we examine the contribution of PPARγ activation and its downstream process. Modified method of scopolamine induced dementia rats were treated with AKBA (5, 10&15 mg/kg,i.p) and Donepezil (2.5 mg/kg,i.p). Scopolamine induced short term spatial, working memory and recognition memory impairment was reversed significantly after AKBA treatment. AKBA administration diminished the Acetylcholine esterase (AchE) activity and preserved brain GABA and glutamate mediated neuronal excitability. Further, gene expression study reveals AKBA ameliorates the memory impairment via activating PPARγ and its downstream regulators, matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9) genes in hippocampus. This study concludes that the treatment with AKBA can be a novel Phyto-molecule of interest for treating dementia via up-regulating hippocampus genes mediated cholinergic activation.
Keywords: 3-O-Acetyl-11-keto-β-boswellic acid, PPARγ, MMP-2, MMP-9, Glutamate, GABA, Dementia
3-O-Acetyl-11-keto-β-boswellic acid; PPARγ; MMP-2; MMP-9; Glutamate; GABA; Dementia.
1. Introduction
Memory is a process of acquisition, consolidation and recognition. Mild cognitive impairment does not affect the day-to-day life, but moderate conditions have a risk of developing dementia. Nevertheless, severe cases undeniably affect the ability to lead an independent life [1]. Studies have recognized that the neuroprotective role of Peroxisome proliferator activated receptors (PPAR) in various cognitive deficit models both in clinical and preclinical studies. Among the four isotypes of PPAR receptors, PPARγ has the highest expression in the central nervous system, chiefly localized in neurons, astrocytes, and glial cells [2, 3].
PPAR-γ agonists protects the learning and memory impairment by improving cytokine release, mitigating mitochondrial stress, release of neurotrophins and enhancing ERK2 activity in hippocampus on Tg2576 mice model of AD [4, 5]. PPARγ also reduces the Aβ load and inflammation, in turn enhances the learning and memory impairment in high fat fed 5XFAD model [5]. Drug candidate like WY14643 potentially enhances the cognitive function through the activation of PPARγ receptor in scopolamine inducted C57BL/6J mice. Nine days administration of pioglitazone against the scopolamine treatment significantly rescued the cholinergic dysfunction and improves the cognitive function [6]. On the other hand, scopolamine treatment potentially inhibits the hippocampal MMP2 and MMP9 release and increases the brain glutamate levels in which inversely affect the acetylcholine release during memory consolidation [7, 8]. Besides, in healthy volunteer, MMP2 and MMP9 gene expression have shown positive association with improvement of working, visual, auditory and verbal cognitive function [9].
Boswellia serrata is a holistic medicinal plant which has a Pentacyclic triterpene compound like AKBA; biologically very active approved by the European Medicines Agency for the treatment of brain edema as an orphan drug [10]. AKBA revealed the potential anti-oxidant and anti-inflammatory activity by suppressing 5-lipoxygenase (5-LOX) and nuclear factor kappa-beta (NF-κB) pathway [11]. AKBA co-treatment with celecoxcib exhibits anti-glutamiergic activity in LPS induced cognitive impairment study [12]. Brain glutamate was significantly reduced in kainic acid provoked excitotoxicity model after AKBA dealing with COX-2 inhibitors [13]. AKBA significantly activates the Nrf 2/HO-1 signaling mechanism during Oxygen glucose deprived condition and provides protection against brain endothelial cell dysfunction [14]. In addition to this, as a result of nuclear factor erythroid-2 (Nrf2) signal inhibition, AKBA improves brain ischemic reperfusion injury in mouse model [15, 16].
Captivatingly, Though AKBA have possible evidence with PPARγ receptor related to cognitive functionality, no study has substantiated yet. Hence, the present study is commenced to clarify the potential role of AKBA on PPARγ receptor and its downstream mechanism in scopolamine induced dementia model.
2. Materials and methods
2.1. Animal and study design
Study design and guidelines followed in this study was approved and monitored by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and Institutional animal ethics committee (IAEC-KMCRET/M.Pharm/2/2019-20). Male Wister albino rats (N = 48) weighing 180–200g were obtained from Biogen laboratory animal facility, Bangalore. n = 8/group. Group I, control (0.5% HPMC, p.o.), Group II, Scopolamine (2 mg/kg,i.p), Group III, Sco + donepezil (2.5 mg/kg, p.o), Group IV, V and VI, Sco + AKBA (5,10 and 15 mg/kg in 0.5% HPMC). Rats were euthanized using ketamine (0.01–0.05 mg/kg).
Duration and dose of AKBA administration (5, 10 15 mg/kg) were selected based on the laboratory of the Vedic Apsen Fitomedicine where 350 mg of dry extract of B. serrata Roxb. ex Colebr–Burseraceae corresponds to 3 mg AKBA was used in inflammatory bowel disease (IBD) [16]. Based on above statement, our preliminary study (acute restrain stress model for 7 days) on (3,10,15 and 20 mg/kg,i.p) AKBA administration showed significant behavioral improvement from the dose of 10, 15 and 20 mg/kg. Moderate Weight loss was found at 20 mg/kg but not in 3, 10, 15 mg/kg dose level, and therefore we focused on AKBA treatment at 5, 10, 15 mg/kg.
2.2. Morris water maze test
Morris water maze (160 cm × 80 cm X 40 cm) is a water filled pool has four identical quadrants maintained at 21 ± 2 °C with a stage immersed from water surface around 1.5 cm placed in dim light area. Four trials have given per day for five days to find the stage which is unchanged during training sessions includes 60 s for locating immersed stage and 20 s to reside on it. If not make the grade, animals will be guided until it learns. Animals were subjected to training session for 5 consecutive days. On Day 6 escape latency time (ELT) to locate the hidden platform in water maze was noted as an index of acquisition or learning. Time spent in the target quadrants and the number of crossings while searching the target quadrant was also noted as index of memory [17].
2.3. Y - maze test
The maze consists of identical, removable sun mica lined chambers arranged in ‘Y’ shape connected to the central chamber. Each arm has a working dimension of approx. 30 cm × 15cm x15 cm with electrifiable grill, and has chamber light or Cue light with indicator, grill charge indicator, rat presence indicator and hinged top. The Central compartment also contains a wire grill. The rats were allowed to explore the ‘Y’ Maze apparatus for 5 min at the start of their training. The rats were then put into the Starting chamber (as per program switch). After 5 s (approx.) shock was applied by switching the unit on or by the rotary program selector till the animal reaches the goal in the illuminated Arm. The animal was then allowed to stay in that arm for the entire inter-trial period. This training continued till the animal attains 9 out of 10 correct choices (An error is counted when the animal enters the wrong compartment during the entire trial). After training the animal was placed in any of the arm and applied shock after 5 s. The time taken by the animal to reach the illuminated arm was noted [18].
2.4. Novel object recognition test
The test consists of three sessions separated by 24 h. In session (habituation); animals were allowed to explore for 10 min. In session 2 (familiarization); two identical objects were placed in the open field and 5 min was given for each animal to interact with the objects. During inter period time (1 h), rats were placed in their home cage. In session 3 (test); one of the identical object was replaced with a novel object differs in shape, color, and texture and the animals were allowed to interact with the object for 10 min. The initial position of the animal facing the objects unchanged throughout the sessions. Contacts with objects defined as when the animal's nose less than 1 cm from the object. No of contact with objects, time to reach the object and time spent in the vicinity of objects were noted [19].
2.5. Acetyl cholinesterase inhibition assay
Acetyl cholinesterase activity was determined in blood plasma according to Elman's method and the developed yellow color due to the cleavage of acetylthiocholine to thiocholine by AchE was read spectro-photometrically at wavelength 412 nm. Units were expressed as nmol/min/mg protein [20].
2.6. Excitatory and inhibitory amino acid estimation in brain tissue
Every 10 mg brain tissue sample from rat brain was homogenised with 0.1N HCL (200μl) in 70% ethanol then transferred to an Eppendorf tube and centrifuged at 4500 rpm/15min in a cooling centrifuge system. The obtained tissue lysate was processed for the estimation of glutamate using L-glutamic acid (10–100 μmol/spot) as standard and for GABA using GABA (5–80 ng/spot) as reference standard at the wavelength of 550 nm using HPTLC system comprising of CAMAG-TLC scanner 3 with linomat-5 applicator [21].
2.7. Quantification of PPAR-γ, MMP-9 and MMP-2 gene expression using RT-qPCR
The mRNA of PPARγ, MMP2 and MMP9 gene expressions were studied using qPCR (Applied Biosystems). From the brain tissue samples, The cells were lysed using TRI reagent and total mRNA was separated and processed to cDNA using conversion kit as per manufacturer's procedure. PCR reactions were run in qPCR (Applied Biosystems, Foster City, CA, USA) system. Primers were added and 35–40 cycle of amplification was initiated after adding the reaction mixtures, at 95 °C for 30s. Primer sequence includes β-actin, sense Primer: 5′-CAACTTTGGCATCGTGGAAG-3′antisense primer: 5′CTGCTTCACCACCTTCTT-3′,MMP-2:sense primer 5′-TGGTGTGGCACCACCGAGGA-3′antisense primer: 5′-CCTTGCCATCGCTTCGGCCA-3′,MMP-9:sense primer 5′-AGCCGGGAACGTATCTGGA-3′, antisense primer:-5′-TGGAAACTCACACGCCAGAAG-3′,PPAR-γ:sense primer: 5′-TAGGTGTGATCTTAACTGTCG-3′, antisense primer 5′–GCATGGTGTAGATGATCTCA-3’. Denaturation at 95 °C for 5 s, and annealing and extension for 30 s. The experimental procedure was triplicated and then the products were separated, visualized and quantified with reference marker gene [22].
2.8. Morphological study
Rat brain hippocampus were collected and fixed with buffered formalin (10%) and perfused with Phosphate buffer solution (1%). Following another 24 h of fixation, brain hippocampus was sectioned and then subjected for paraffinization and de-paraffinization process. There after hippocampus sections were stained with hematoxylin and Eosin markers, and examined microscopically at the magnification of 40x.
2.9. Statistical analysis
Statistics (N = 6, Mean ± SEM) were performed based on one way ANOVA followed by post hoc analysis Turkey's multiple comparison test using prism software 5.0. Also, we carried out correlation coefficient analysis to verify the possible relationship between the two liner dependent variables. Data expressed, If R2, >0.9 or more considered as a very high degree of positive correlation. If R2, > -0.9 or more considered as a very high degree of negative correlation between the dependent variables.
3. Results
3.1. Effect of AKBA on spatial learning and memory performance
Short term spatial learning and memory were examined by Morris water maze test. Scopolamine induction markedly increased the retention time [F (5, 42) = 22.3, p < 0.001] as compared to control (Figure 1A). Donepezil 2.5 mg/kg markedly [F (5, 42) = 17.24, P < 0.001] reduced the retention time in comparison with scopolamine. Treatment with AKBA at 5 mg/kg [F (5, 42) = 9.11, P < 0.05], 10 mg/kg [F 5, 42) = 12.31, P < 0.01] and 15 mg/kg [F (5, 42) = 15.72,P < 0.001] significantly reduced the retention time as compared to scopolamine. Treatment with scopolamine resulted in significantly decreased the time spent as compared to control in target quadrant [F (5, 42) = 19.48, P < 0.001] (Figure 1B). AKBA treatment at 10 mg/kg [F (5, 42) = 7.03, P < 0.05] and 15 mg/kg [F (5, 42) = 13.70, P < 0.01] significantly increased the time spent for scopolamine. Number of crossings around the target quadrant is decreased for scopolamine group when compared to control [F (5, 42) = 9.56, P < 0.01]. For donepezil [F (5, 42) = 8.11, P < 0.01] in comparison with scopolamine group. 10 mg/kg [F (5, 42) = 5.63, P < 0.05] and 15 mg/kg [F (5, 42) = 6.05, P < 0.01] of AKBA administered rats exhibited the increased number of crossings compared to scopolamine. There is no significant difference for AKBA at 5 mg/kg on time spent and crossings when compared to scopolamine group (Figure 1C).
Figure 1.
Effect of AKBA on Morris water maze performance and Y Maze test. A) Retention time B) Time spent in target quadrant C) No of Crossings D) Escape latency time. (N = 8), Statistical analysis was carried out in one-way ANOVA followed by post hoc analysis Turkey's multiple comparison test using prism 5.0. Statistical significance ∗P < 0.05 Vs control; #P < 0.05 Vs scopolamine treated group.
3.2. Effect of AKBA on short-term working memory performance
Scopolamine treated rats showed [F (5, 42) = 15.6, P < 0.001] significant increase of escape latency time in comparison to control. In turn, Treatment with Donepezil [F (5, 42) = -13.12, P < 0.001] and AKBA at 5 mg/kg, [F (5, 42) = 6.83, P < 0.05], 10 mg/kg [F (5, 42) = 8.45, P < 0.01] and 15 mg/kg [F (5, 42) = -9.79, P < 0.01] markedly decreased the escape latency time when compared to scopolamine group (Figure 1D).
3.3. Effect of AKBA on recognition memory performance
Scopolamine treated rats significantly decreased the number of contacts with novel object [F (5, 42) = 13.12, P < 0.01], increased with familiar object [F (5, 42) = 26.61, P < 0.001] in comparison to control (Figure 2A). Compared to scopolamine group, number of contacts were found to be increased with novel object [F (5, 42) = 15.81, P < 0.001] and decreased with familiar object [F (5, 42) = 21.02, P < 0.001] for donepezil treated group. AKBA (5,10 and 15 mg/kg) significantly increased the number of contacts with novel object [F (5, 42) = 10.2, P < 0.05] and significantly decreased with familiar object [F (5, 42) = 17.83, P < 0.05) compared to scopolamine treatment. Scopolamine induction significantly reduced the time spent with the novel object [F (5, 42) = 36.07, P < 0.001] and increased with familiar object [F (5, 42) = 54.12, P < 0.001] as compared to control group. Donepezil increased the time spent with novel object [F (5, 42) = 30.46, P < 0.01] and decreased with familiar object [F (5, 42) = 42.62, P < 0.001] as compared to scopolamine. AKBA (5,10and 15 mg/kg) treatment significantly augment the time spent with novel object [F (5, 42) = 16.84, P < 0.05) and significantly lessen the time spent with familiar object [F (5, 42) = 22.47, P < 0.05] were observed as comparable to scopolamine (Figure 2B). The time taken by the scopolamine treated rats to reach the novel object [F (5, 42) = 24.05, P < 0.001] was less than that of familiar object [F (5, 42) = 37.73, P < 0.001] (Figure 2C). For Donepezil treated group, time to reach the novel object [F (5, 42) = 19.42, P < 0.001] was less than that of familiar object as compared to scopolamine group [F (5, 42) = 28.71, P < 0.01]. AKBA (5, 10 and 15 mg/kg) treated group takes less time to reach the novel object [F (5, 42) = 15.45, P < 0.01) than familiar object [F (5, 42) = 12.06, P < 0.05] when compared to scopolamine (Figure 2C).
Figure 2.
Effect of AKBA on novel object recognition test. A) No of contacts B) Time spent C) Time to reach. (N = 8), Statistical analysis was carried out in one-way ANOVA followed by post hoc analysis Turkey's multiple comparison test using prism 5.0. Statistical significance ∗P < 0.05 Vs Control; #P < 0.05 Vs Scopolamine treated group.
3.4. Effect of AKBA on acetylcholine esterase activity
In comparison to control group, scopolamine group significantly increased the rate of acetylcholine esterase activity [F (5, 42) = 7.23, P < 0.001] (Figure 3A). Donepezil [F (5, 42) = 5.07, P < 0.01] decreased the rate of enzyme activity in comparison with scopolamine treated group. Likewise, Administration of AKBA at 5 mg/kg, [F (5, 42) = 2.31, P < 0.05]. 10 mg/kg [F (5, 42) = 4.02, P < 0.01] and 15 mg/kg [F (5, 42) = 4.83, P < 0.01] showed diminution in rate of enzyme activity as compared to scopolamine.
Figure 3.
Effect of AKBA on plasma Acetyl cholinesterase activity, brain glutamate and GABA level. A) Enzyme activity B) Glutamate level C) GABA level. (N = 8), Statistical analysis was carried out in one-way ANOVA followed by post hoc analysis Turkey's multiple comparison test using prism 5.0. Statistical significance, ∗P < 0.05 Vs Control; #P < 0.05 Vs Scopolamine treated group.
3.5. Effect of AKBA on brain excitatory and inhibitory amino acids
Scopolamine treated group significantly increased the glutamate level when compared to control [F (5, 42) = 13.2, P < 0.001] (Figure 3B). Treatment with Donepezil [F (5, 42) = 10.36, P < 0.01] decreased the glutamate level in comparison with scopolamine. AKBA (10 and 15 mg/kg), [F (5, 42) = 6.71, P < 0.05] and [F (5, 42) = 6.94, P < 0.05] treatment diminished the glutamate level as compared to scopolamine. On the other hand, scopolamine significantly decreased the GABA level when compared to control [F (5, 42) = 16.2, P < 0.001]. In contrast, Treatment with Donepezil [F (5, 42) = 14.72, P < 0.001] markedly increased the GABA level in comparison with scopolamine treated group. Similarly, AKBA (10 and 15 mg/kg) [F (5, 42) = 8.36, P < 0.05] and [F (5, 42) = 12.47, P < 0.01] treatment increased the GABA level compared with scopolamine (Figure 3C).
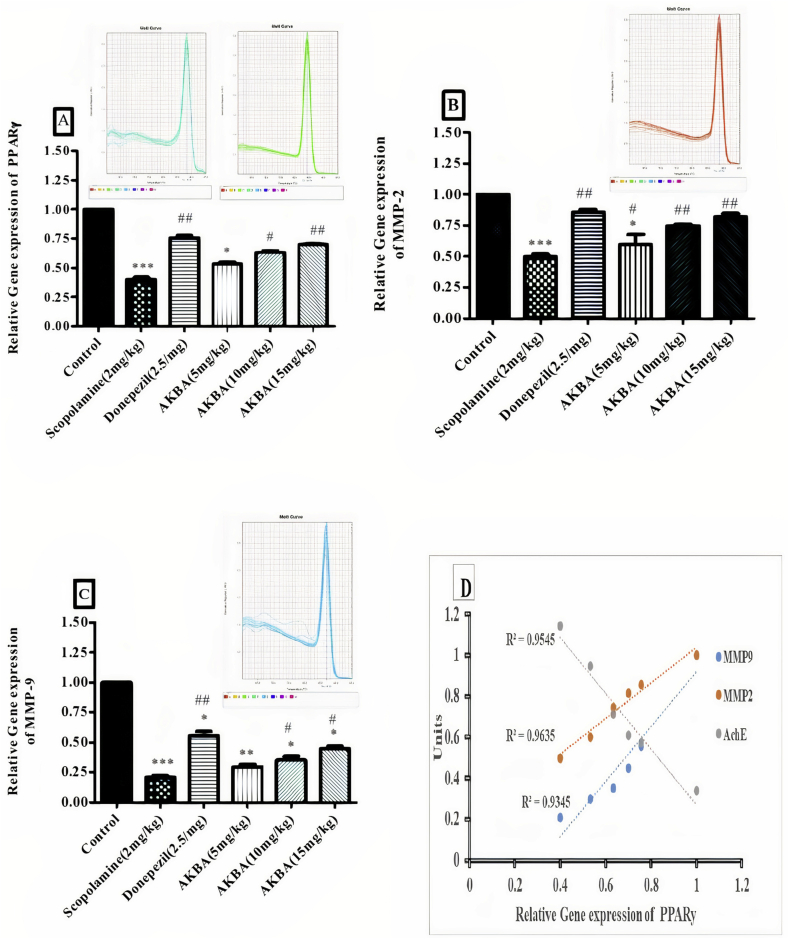
3.6. Effect of AKBA on PPARγ, MMP-2 and MMP-9 relative gene expression
Scopolamine treatment significantly decreases the relative gene expression of PPARγ as compared to control [F (5, 12) = 21.6, P < 0.001] (Figure 4A) When compared to scopolamine, Donepezil treatment increased the relative gene expression level of PPARγ [F (5, 12) = 17.25, P < 0.01]. Similarly, AKBA treatment at 10 mg/kg and 15 mg/kg significantly enhanced the gene expression level of PPARγ [F (5, 12) = 10.32, P < 0.05, F (5, 12) = 13.51 P < 0.01] when compared to scopolamine treated group. Treatment with scopolamine reduced the relative gene expression of MMP-2 compared with control [F (5, 12) = 13.52, P < 0.001] (Figure 4B). Donepezil treatment markedly increased the relative gene expression level [F (5, 12) = 11.36, P < 0.01] in comparison to scopolamine. In the same way, Treatment of AKBA (5, 10 &15 mg/kg) increased the relative gene expression level [F (5, 12) = 6.2, P < 0.05, F (5, 12) = 7.83, P < 0.01 and F (5, 12) = 8.43, P < 0.001] compared with scopolamine. In addition to this, scopolamine treatment exhibited reduction in the relative gene expression of MMP-9 when compared to control group [F (5, 12) = 15.37, P < 0.001] (Figure 4C). In comparison with scopolamine treated group, Donepezil treatment increased the relative gene expression of MMP-2 [F (5, 12) = 8.59, P < 0.01]. AKBA treatment (10 mg/kg and 15 mg/kg) shows increased in gene expression level [F (5, 12) = 6.47, P < 0.05, F (5, 12) = 6.91, P < 0.05] when compared to scopolamine treated group.
Figure 4.
Effect of AKBA on relative gene expression of A) PPARγ, B) MMP-2 and C) MMP9. Statistical analysis was carried out in one-way ANOVA followed by post hoc analysis Turkey's multiple comparison tests using prism 5.0. Statistical significance, ∗∗∗P < 0.001 Vs Control. ##P < 0.01 Vs Scopolamine treated group. D) Correlation analysis, PPARγ expression Vs AchE, PPARγ expression Vs MMP-2 expression, PPARγ expression Vs MMP-9 expression. R2 > 0.9 shows significant level.
3.7. Correlation analysis
In Correlation coefficient study, association of PPARγ with AchE activity, MMP2 and MMP9 gene expression were examined. Hippocampal PPARγ gene expression showed higher negative correlation with AchE activity (R2 = 0.954), In contrast, MMP2 (R2 = 0.963) and MMP9 (R2 = 0.934) revealed positive correlation with PPARγ gene expression in cognitive functionality assessment (Figure 4D).
3.8. Effect of AKBA on hippocampal histology
Scopolamine treated rats shown significant neuronal changes compared to control group of rat hippocampus CA1 region. Hippocampal morphology illustrates prominent cell shrinkage, necrosis, mild hemorrhage and gliosis in scopolamine rats. On contrary, Donepezil group showed mild gliosis and hemorrhage were observed, but no cell shrinkage, necrosis was present. Similar pattern of histological changes was observed in AKBA (10 & 15 mg/kg) treated group as compared to CUMS. In AKBA 5 mg/kg, showed mild cell shrinkage and gliosis were observed compared to Scopolamine group (Figure 5).
Figure 5.
Effect of AKBA on Hippocampus Morphology, H&E staining with 40X magnification. A) Control, B) Scopolamine C) Donepezil, D) AKBA 5 mg/kg, E) AKBA 10 mg/kg, F) AKBA 15 mg/kg.
4. Discussion
The present study scrutinized the role of AKBA on modified scopolamine induced dementia model in rodents by observing behavioral, biochemical and gene expression pattern through the conformational changes on PPARγ receptor and their downstream mechanism and by the neuronal staining techniques in hippocampus CA1 region. Scopolamine induced cognitive destruction in rodent is meticulously resemblance to clinical symptoms of early and late sporadic AD. Moreover, scopolamine induction depicts, not only acquired learning and memory, also demonstrates the recognition memory condition as well [23]. Hippocampus based spatial learning and memory sequence was experimented in MWM platform. Animals routing to hidden platform improves the ability to learn the task which is in direction with the hippocampus CA1, CA3 and DG architect and its neuronal actions [24]. During the seven days trial, retention time was observed and gradual reduction on day-to-day trial was noted, this shows that continuous exposure of identical activity may reduce the retention time thereby accelerating the learning and memory performance, while augmentation of time spent and platform crossing indicates improvement in reference memory [25]. Administration of AKBA drastically improved the spatial learning and reference memory performance by reducing retention time, increasing time spent and platform crossing to the target zone.
Increased ELT and any escape to non-safe zones were regarded as error which represents the impairment of short-term memory in Y maze task [18]. Scopolamine exhibited marked increase in escape latency time to safe zone, further confirmed by more non safe zone entry indicates short term memory deficits. This study signifies that different dose of AKBA administration reversed the scopolamine induced short term memory deficit in dose dependent manner by increasing the number of safe zone entry and by reducing the escape latency time to safe zone than the non-safe zone area. Furthermore, the discriminating ability of rodents were analyzed by the NOR test [26]. AKBA treatment potentiates the remembrance capacity towards similarity between the two objects than the scopolamine treated rats. Treatment with AKBA increased the time spent and time to reach the novel object was found to be more than the familiar object, depicts that AKBA potentiate the process of consolidation and retrieval effectively in rodents. Likely, Donepezil treated animals expressed better connectivity pattern towards the novel object approachability and contact time than the familiar objects. This in connection with treatment group showed significant increase in number of contacts with novel object, represents course of object information encoding process is well established which is lack in scopolamine treated rats.
Abnormality in cholinergic neuron is very crucial to amnesia, dementia and AD like pathological condition [27]. Scopolamine is a short acting cholinergic muscarinic receptor antagonist cause cerebral atrophy and degeneration of cholinergic neurons thereby induces cholinergic dysfunction by decreasing acetylcholine release and choline acetyltransferase activity, and by increasing acetylcholine esterase and butyrylcholine esterase activities [28, 29, 30]. Stimulation of PPARγ receptor resulted in the activation of the cholinergic neurons in cortex and hippocampus, there by increasing the acetylcholine release and decreasing AchE activity and increasing ChAT activity [6]. AKBA significantly reversed the scopolamine induced elevated AchE activity which in turn increased the acetylcholine release. In addition to this, AKAB treatment significantly improved the mRNA of PPARγ gene expression in CA1 region. In contrast, PPARγ expression was significantly lessen in scopolamine treated animals where the AchE activity was considerably high. Previous study demonstrate that hippocampus PPARγ receptor activation improves short term spatial learning and memory response in rodents by decreasing the AchE activity [31]. From the aforesaid statement, we speculate that improvement of different type of learning and memory activity in MWM, Y maze task and NORT after AKBA treatment is strongly associated to PPARγ receptor activation.
Central dogma of nervous system is neurons and astrocytes maintain the glutamate concentration at considerable level to keep up the synaptic integrity and to prevent the excitotoxicity contributes to cognition and other therapeutic functions [32]. Enhancement of the glutamatergic signal may cause adverse effects on memory formation, since elevated level of glutamate considers as neurotoxic leads to brain deterioration [33]. On the assessment, AKBA significantly reduced the glutamate level induced by scopolamine. This observation strongly supports the earlier statement, AKBA significantly inhibits glutamate induced excitotoxicity in PC12 and N2a neuronal cells. In depressive rats, AKBA potentially inhibits the glutamic acid decarboxylase (GAD) enzyme activation which is involved in the course of glutamate synthesis process [21, 34]. Moreover, AKBA remarkably increased the GABA content against scopolamine treatment, which is in line with previous report; cognitive impairment induced by scopolamine is coupled with GABA reduction in the cortical and hippocampus region. However, GABA is an inhibitor neurotransmitter that prevents the excitability of neuronal cells [17]. Adding evidence, Rosiglitazone, a PPARγ agonist remarkably increases the elimination of glutamate from neuronal cells through the activation of excitatory amino acid transporters (EAAT2) in OGD model [35].
In the course of memory formation, MMP2 and MMP9 play a decisive role in consolidation and retrieval process. Meanwhile, controversial report has also been placed between MMPs and cognitive functions, stating that accumulation of Beta amyloid (Aβ) protein in astrocytes stimulate the MMP-2&9 gene expression in AD [36]. On the contrary, administration of MMP-2&9 inhibitors deteriorate the acquired memory performance in MWM task and worsen the conditioned place preference reaction in nicotinate rats [37]. Another study of patients with vascular cognitive impairment have displayed the reduced MMP2 index range in CSF [38]. Furthermore, depressive patients observed with poor cognitive performance which is negatively correlated to mRNA expression of MMP-2, MMP-9 and TIMP-2 genes and its proteins that directly correlates to plasma acetylcholine level [9]. In this study, scopolamine induction declines the mRNA of MMP2 and MMP9 genes in hippocampus which was reversed significantly after AKBA administration. On treatment, AKBA dose dependently improve the expression of mRNA of PPARγ gene, as well as MMP2 and MMP9 genes against scopolamine treatment. However, our study report made obvious that the activation of PPARγ gene positively associated to MMP2 and MMP9 gene expression in memory formation.
Neurofilaments are essential to maintain the structural integrity, rigidity and functions of neurons observed in many neurodegenerative events. Disruption of their architect associate to abnormal hippocampal plasticity led to secondary damage to cytoskeleton of axon and its internal role. Studies also demonstrate that disarrangements of hippocampus neurons could affect the long term potentiation (LTP), working and special memory activity [39]. Our study on rat hippocampus CA1 region has proved that the scopolamine group develops cell shrinkage, reactive gliosis, extensive areas of necrosis and hemorrhage than the normal rats. These histological changes were markedly attenuated in AKBA treated groups dose dependently. This morphological changes by AKBA, might be due to the activation of PPARγ receptor in hippocampus where the expression of PPARγ mRNA gene found more, also it would be due to the suppression of glutamate release and to maintain the excitability of neuronal cells in hippocampus region [27].
From the study report, AKBA showed potent neuroprotective action against scopolamine induced hippocampal deterioration mediated cognitive impairment. Further AKBA impersonates PPARγ receptor like action on improving cognitive function through the activation of PPARγ gene and its downstream regulators. Therefore, we conclude that the molecular mechanism of AKBA strongly associated to PPARγ receptor mediated cholinergic activation in cognitive function.
Declarations
Author contribution statement
Venkatesh Gunasekaran: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jinu Avarachan: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Anitta Augustine: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Abdul Khayum: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Arivukkarasu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We acknowledge the Management of KMCH College of Pharmacy and PSG College of Pharmacy for constant support to carry out research work. We thank Mr. Saravana for plagiarism and grammar check. Also, we thank Arjuna Natural limited, Kerala, India. for rendering chemical support.
References
- 1.Kales H.C., Lyketsos C.G., Miller E.M., Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: an international Delphi consensus. Int. Psychogeriatr. 2019;31:83–90. doi: 10.1017/S1041610218000534. [DOI] [PubMed] [Google Scholar]
- 2.Moreno S., Farioli-Vecchioli S., Cerù M. Immunolocalization of peroxisome proliferator-activated receptors and retinoid x receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 3.Beheshti F., Hosseini M., Hashemzehi M., Soukhtanloo M., Khazaei M., Naser Shafei M. The effects of PPAR-γ agonist pioglitazone on hippocampal cytokines, brain-derived neurotrophic factor, memory impairment, and oxidative stress status in lipopolysaccharidetreated rats, Iran. J. Basic Med. Sci. 2019 doi: 10.22038/ijbms.2019.36165.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denner L.A., Rodriguez-Rivera J., Haidacher S.J., Jahrling J.B., Carmical J.R., Hernandez C.M., Zhao Y., Sadygov R.G., Starkey J.M., Spratt H., Luxon B.A., Wood T.G., Dineley K.T. Cognitive enhancement with Rosiglitazone links the hippocampal PPAR and ERK MAPK signaling pathways. J. Neurosci. 2012;32:16725–16735. doi: 10.1523/JNEUROSCI.2153-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medrano-Jiménez E., Jiménez-Ferrer Carrillo I., Pedraza-Escalona M., Ramírez-Serrano C.E., Álvarez-Arellano L., Cortés-Mendoza J., Herrera-Ruiz M., Jiménez-Ferrer E., Zamilpa A., Tortoriello J., Pedraza-Alva G., Pérez-Martínez L. Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-γ-dependent mechanism. J. Neuroinflammation. 2019;16:143. doi: 10.1186/s12974-019-1515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang G.Q., Tang S.S., Jiang L.Y., Hong H., Li Q., Wang C., Wang X.Y., Zhang T.T., Yin L. PPAR γ agonist pioglitazone improves scopolamine-induced memory impairment in mice. J. Pharm. Pharmacol. 2012;64:589–596. doi: 10.1111/j.2042-7158.2011.01432.x. [DOI] [PubMed] [Google Scholar]
- 7.Moosavi M., SoukhakLari R., Moezi L., Pirsalami F. Scopolamine-induced passive avoidance memory retrieval deficit is accompanied with hippocampal MMP2, MMP-9 and MAPKs alteration. Eur. J. Pharmacol. 2018;819:248–253. doi: 10.1016/j.ejphar.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Garabadu D., Sharma M. Eugenol attenuates scopolamine-induced hippocampal cholinergic, glutamatergic, and mitochondrial toxicity in experimental rats. Neurotox. Res. 2019;35:848–859. doi: 10.1007/s12640-019-0008-6. [DOI] [PubMed] [Google Scholar]
- 9.Bobińska K., Szemraj J., Gałecki P., Talarowska M. The role of MMP genes in recurrent depressive disorders and cognitive functions. Acta Neuropsychiatr. 2016;28:221–231. doi: 10.1017/neu.2015.72. [DOI] [PubMed] [Google Scholar]
- 10.Skarke C., Kuczka K., Tausch L., Werz O., Rossmanith T., Barrett J.S., Harder S., Holtmeier W., Schwarz J.A. Increased bioavailability of 11-Keto-β-Boswellic acid following single oral dose Frankincense extract administration after a standardized meal in healthy male volunteers: modeling and simulation considerations for evaluating drug exposures. J. Clin. Pharmacol. 2012;52:1592–1600. doi: 10.1177/0091270011422811. [DOI] [PubMed] [Google Scholar]
- 11.Ding Y., Qiao Y., Wang M., Zhang H., Li L., Zhang Y., Ge J., Song Y., Li Y., Wen A. Enhanced neuroprotection of acetyl-11-keto-β-boswellic acid (AKBA)-Loaded O-carboxymethyl chitosan nanoparticles through antioxidant and anti-inflammatory pathways. Mol. Neurobiol. 2016;53:3842–3853. doi: 10.1007/s12035-015-9333-9. [DOI] [PubMed] [Google Scholar]
- 12.Sayed A.S., El Sayed N.S.E.D. Co-administration of 3-acetyl-11-keto-beta-boswellic acid potentiates the protective effect of celecoxib in lipopolysaccharide-induced cognitive impairment in mice: possible implication of anti-inflammatory and antiglutamatergic pathways. J. Mol. Neurosci. 2016 doi: 10.1007/s12031-016-0734-7. [DOI] [PubMed] [Google Scholar]
- 13.Bishnoi M., Patil C.S., Kumar A., Kulkarni S.K. Co-administration of acetyl-11-keto-β-boswellic acid, a specific 5-lipoxygenase inhibitor, potentiates the protective effect of COX-2 inhibitors in kainic acid-induced neurotoxicity in mice. Pharmacology. 2007 doi: 10.1159/000097627. [DOI] [PubMed] [Google Scholar]
- 14.Sadeghnia H.R., Arjmand F., Ghorbani A. Neuroprotective effect of Boswellia serrata and its active constituent acetyl 11-keto-β-boswellic acid against oxygen-glucose-serum deprivation-induced cell injury. Acta Pol. Pharm. Drug Res. 2017 [PubMed] [Google Scholar]
- 15.Ahmad S., Khan S.A., Kindelin A., Mohseni T., Bhatia K., Hoda M.N., Ducruet A.F. Acetyl-11-keto-β-boswellic acid (AKBA) attenuates oxidative stress, inflammation, complement activation and cell death in brain endothelial cells following OGD/reperfusion. Neuro. Mol. Med. 2019 doi: 10.1007/s12017-019-08569-z. [DOI] [PubMed] [Google Scholar]
- 16.Krieglstein C.F., Anthoni C., Rijcken E.J.M., Laukötter M., Spiegel H.-U., Boden S.E., Schweizer S., Safayhi H., Senninger N., Schürmann G. Acetyl-11-keto-β-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int. J. Colorectal Dis. 2001;16:88–95. doi: 10.1007/s003840100292. [DOI] [PubMed] [Google Scholar]
- 17.El-Marasy S.A.A., Abd-Elsalam R.M., Ahmed-Farid O.A. Ameliorative effect of silymarin on scopolamine-induced dementia in rats, open access maced. J. Med. Sci. 2018;6:1215–1224. doi: 10.3889/oamjms.2018.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ru M., Liu H. Association between Y-maze acquisition learning and major histocompatibility complex class II polymorphisms in mice. BioMed Res. Int. 2018;2018:1–6. doi: 10.1155/2018/6381932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antunes M., Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognit. Process. 2012 doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961 doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan M., Saravana Babu C., Justin A., Shanthakumari S. Elucidation of neuroprotective role of endogenous GABA and energy metabolites in middle cerebral artery occluded model in rats. Indian J. Exp. Biol. 2012 [PubMed] [Google Scholar]
- 22.Darshit B.S., Ramanathan M. Activation of AKT1/GSK-3β/β-catenin–TRIM11/survivin pathway by novel GSK-3β inhibitor promotes neuron cell survival: study in differentiated SH-SY5Y cells in OGD model. Mol. Neurobiol. 2016;53:6716–6729. doi: 10.1007/s12035-015-9598-z. [DOI] [PubMed] [Google Scholar]
- 23.Bhuvanendran S., Kumari Y., Othman I., Shaikh M.F. Amelioration of cognitive deficit by embelin in a scopolamine-induced alzheimer’s disease-like condition in a rat model. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poucet B., Save E., Lenck-Santini P.-P. Sensory and memory properties of hippocampal place cells. Rev. Neurosci. 2000;11 doi: 10.1515/revneuro.2000.11.2-3.95. [DOI] [PubMed] [Google Scholar]
- 25.Venkatesh G., Sankar V., Ramanathan M. Molecular mechanism of tuberoinfundibular peptide of 39 on glucocorticoid receptor mediated glutamate/GABA imbalance and cerebral abnormalities against cognitive deficit model. J. Pharm. Pharmacol. 2019;71:996–1006. doi: 10.1111/jphp.13085. [DOI] [PubMed] [Google Scholar]
- 26.Eagle A.L., Fitzpatrick C.J., Perrine S.A. Single prolonged stress impairs social and object novelty recognition in rats. Behav. Brain Res. 2013;256:591–597. doi: 10.1016/j.bbr.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson L., Platt B., Riedel G. Involvement of the cholinergic system in conditioning and perceptual memory. Behav. Brain Res. 2011 doi: 10.1016/j.bbr.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Safar M.M., Arab H.H., Rizk S.M., El-Maraghy S.A. Bone marrow-derived endothelial progenitor cells protect against scopolamine-induced alzheimer-like pathological aberrations. Mol. Neurobiol. 2016 doi: 10.1007/s12035-014-9051-8. [DOI] [PubMed] [Google Scholar]
- 29.Xu Q.-Q., Xu Y.-J., Yang C., Tang Y., Li L., Cai H.-B., Hou B.-N., Chen H.-F., Wang Q., Shi X.-G., Zhang S.-J. Sodium tanshinone IIA sulfonate attenuates scopolamine-induced cognitive dysfunctions via improving cholinergic system. BioMed Res. Int. 2016;2016:1–9. doi: 10.1155/2016/9852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song S.H., Choi S.M., Kim J.E., Sung J.E., Lee H.A., Choi Y.H., Bae C.J., Choi Y.W., Hwang D.Y. α-Isocubebenol alleviates scopolamine-induced cognitive impairment by repressing acetylcholinesterase activity. Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Hajjar T., Meng G.Y., Rajion M.A., Vidyadaran S., Othman F., Farjam A.S., Li T.A., Ebrahimi M. Omega 3 polyunsaturated fatty acid improves spatial learning and hippocampal Peroxisome Proliferator Activated Receptors (PPARα and PPARγ) gene expression in rats. BMC Neurosci. 2012;13:109. doi: 10.1186/1471-2202-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tapiero H., Mathé G., Couvreur P., Tew K. Arginine. Biomed. Pharmacother. 2002;56:439–445. doi: 10.1016/s0753-3322(02)00284-6. [DOI] [PubMed] [Google Scholar]
- 33.Afshari A.R., Fanoudi S., Rajabian A., Sadeghnia H.R., Mollazadeh H., Hosseini A. Potential protective roles of phytochemicals on glutamateinduced neurotoxicity: a review, Iran. J. Basic Med. Sci. 2020 doi: 10.22038/ijbms.2020.43687.10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajabian A., Sadeghnia H.R., Hosseini A., Mousavi S.H., Boroushaki M.T. 3-Acetyl-11-keto-β-boswellic acid attenuated oxidative glutamate toxicity in neuron-like cell lines by apoptosis inhibition. J. Cell. Biochem. 2020;121:1778–1789. doi: 10.1002/jcb.29413. [DOI] [PubMed] [Google Scholar]
- 35.Romera C., Hurtado O., Mallolas J., Pereira M.P., Morales J.R., Romera A., Serena J., Vivancos J., Nombela F., Lorenzo P., Lizasoain I., Moro M.A. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARγ target gene involved in neuroprotection. J. Cerebr. Blood Flow Metabol. 2007;27:1327–1338. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto M., Takagi Y., Aoki T., Hayase M., Marumo T., Gomi M., Nishimura M., Kataoka H., Hashimoto N., Nozaki K. Tissue inhibitor of metalloproteinases protect blood—brain barrier disruption in focal cerebral ischemia. J. Cerebr. Blood Flow Metabol. 2008;28:1674–1685. doi: 10.1038/jcbfm.2008.59. [DOI] [PubMed] [Google Scholar]
- 37.Natarajan R., Harding J.W., Wright J.W. A role for matrix metalloproteinases in nicotine-induced conditioned place preference and relapse in adolescent female rats. J. Exp. Neurosci. 2013;7 doi: 10.4137/JEN.S11381. JEN.S11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Candelario-Jalil E., Thompson J., Taheri S., Grossetete M., Adair J.C., Edmonds E., Prestopnik J., Wills J., Rosenberg G.A. Matrix metalloproteinases are associated with increased blood–brain barrier opening in vascular cognitive impairment. Stroke. 2011;42:1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotow T. Neurofilaments in health and disease. Med. Electron. Microsc. 2000 doi: 10.1007/s007950000019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.