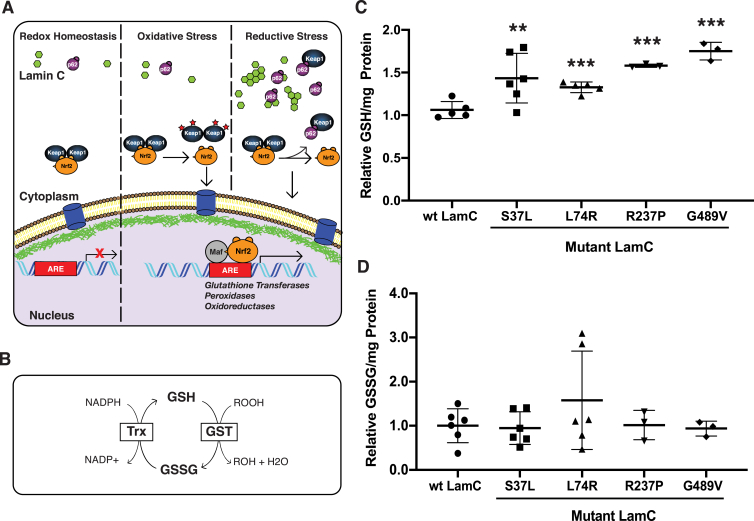
Fig. 3.
Mutant LamC alters redox homeostasis. (A) A model illustrating the Nrf2/Keap1 pathway under redox homeostasis, oxidative stress, and reductive stress. Under redox homeostasis, the pathway is inactive, with Nrf2 (orange) being rapidly degraded through the ubiquination pathway. Under oxidative stress, cysteine residues in Keap1 are oxidized, preventing Nrf2 binding. This permits Nrf2 to translocate into the nucleus, form a heterodimer with small Maf (grey) at antioxidant responsive elements (AREs), and activate Nrf2 target genes. Under reductive stress, cytoplasmic aggregates (green) cause increased levels of the autophagy chaperone p62 (purple). p62 competes with Nrf2 for Keap1 binding, leading to free Nrf2 in the cytoplasm, which translocates into the nucleus, forms a heterodimer with small Maf (grey) at antioxidant responsive elements (AREs), and activates antioxidant genes. (B) A diagram of the pathway for production of reduced glutathione (GSH) and oxidized glutathione (GSSG) in Drosophila is shown. (C) The relative amount of GSH per milligram of total protein in third instar larval muscle expressing either wild-type or mutant LamC is shown. Each point represents an independent biological replicate. (D) The relative amount of GSSG per milligram of total protein in third instar larval muscle expressing either wild-type or mutant LamC is shown. Concentrations were determined via a GSH→GSSG recycling assay using three to five independent biological samples from separate genetic crosses. An ANOVA analysis was performed to test for significance: **p < 0.01; ***, p < 0.001. LamC L74R showed the most variability among the independent biological replicates. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)