Abstract
Unravelling the environmental factors driving species distribution and abundance is crucial in ecology and conservation. Both climatic and land cover factors are often used to describe species distribution/abundance, but their interrelations have been scarcely investigated. Climatic factors may indeed affect species both directly and indirectly, e.g., by influencing vegetation structure and composition. We aimed to disentangle the direct and indirect effects (via vegetation) of local temperature on bird abundance across a wide elevational gradient in the European Alps, ranging from montane forests to high-elevation open areas. In 2018, we surveyed birds by using point counts and collected fine-scale land cover and temperature data from 109 sampling points. We used structural equation modelling to estimate direct and indirect effects of local climate on bird abundance. We obtained a sufficient sample for 15 species, characterized by a broad variety of ecological requirements. For all species we found a significant indirect effect of local temperatures via vegetation on bird abundance. Direct effects of temperature were less common and were observed in seven woodland/shrubland species, including only mountain generalists; in these cases, local temperatures showed a positive effect, suggesting that on average our study area is likely colder than the thermal optimum of those species. The generalized occurrence of indirect temperature effects within our species set demonstrates the importance of considering both climate and land cover changes to obtain more reliable predictions of future species distribution/abundance. In fact, many species may be largely tracking suitable habitat rather than thermal niches, especially among homeotherm organisms like birds.
Keywords: Elevational gradient, Bird ecology, Thermal niche, European Alps, High-elevation species
Introduction
Climate and land-use/land-cover strongly affect the distribution and abundance of species and many ecosystems functions (e.g., Sala et al., 2000). To correctly understand the impact of global change, and in particular of climate and land-use modification, unravelling the environmental factors determining species distribution and abundance is crucial (Rodríguez et al., 2007; Péron & Altwegg, 2015; Engler et al., 2017). While this is a key issue across ecosystems and realms (Mantyka-Pringle, Martin & Rhodes, 2012), for some groups, such as birds (e.g., Jongsomjit et al., 2013), and some environments, such as mountains (Dirnböck, Essl & Rabitsch, 2011), disentangling those effects is of utmost importance to address urgent conservation challenges (Chamberlain et al., 2013). Unfortunately, studies explicitly addressing both land-use and climate change effects are relatively scarce (e.g., Pearce-Higgins et al., 2015), and there are few studies that have really tried to quantitatively partition the interactive effects of climate and land-use on target species.
Mountains are characterized by a strong variation across short distances of abiotic factors such as temperature, precipitation, atmospheric pressure, slope and exposure. This leads to a high variety of habitats and species occurring within relatively small areas and stratified across elevational gradients (Körner & Ohsawa, 2006; Cadena et al., 2012). This often results also in patchily distributions of species that are strictly connected to very specific elevation ranges, and also in high endemism levels (Ruggiero & Hawkins, 2008; Cadena et al., 2012). Mountain ecosystems are consequently extremely valuable in terms of biodiversity and conservation (Myers et al., 2000; Körner & Ohsawa, 2006; Boyle & Martin, 2015), but species specialist of those ecosystems are highly threatened by climate change (e.g., Goodenough & Hart, 2013; Brambilla et al., 2017; De Gabriel Hernando et al., 2021), because of their adaptation to local conditions (Cheviron & Brumfield, 2012). Among birds, species distribution/abundance along elevational gradients have been investigated in several studies (e.g., Chamberlain et al., 2016; Frey, Hadley & Betts, 2016; Elsen et al., 2017; Jähnig et al., 2020), considering factors such as local climate, vegetation characteristics and topography as explanatory variables. Studies on factors driving occurrence or abundance along environmental gradients provide highly valuable information, but the interplay between climate and vegetation in determining mountain bird distribution/abundance is still unclear. In fact, these factors are usually considered independently in data analyses, without estimating the indirect effects deriving from the causal relationships between climate and vegetation (but see Duclos, DeLuca & King (2019) for a set of forest bird species). As a consequence, the degree to which climate impacts on species’ occurrence or abundance directly (by affecting, e.g., thermoregulation, nest site selection, breeding phenology; Martin, 2001; Leech & Crick, 2007; Barnagaud et al., 2011; Bison et al., 2020) or indirectly, through e.g., its influence on vegetation structure and composition, is largely unknown. This is a highly relevant issue for ecology and conservation, especially because it is key to predict climate change effects on bird populations: species distribution changes and elevational shifts indeed are often interpreted as a thermal niche tracking but, if climate effects are mostly or exclusively indirect, such distribution changes will be rather a consequence of habitat tracking. In this case, predictive distribution modelling should account for future changes in vegetation structure and composition due to climate change and other (possibly interacting) factors such as human land use. Knowledge about the relative magnitude of direct and indirect climate effects would also allow to propose more effective conservation strategies and management measures, or to improve the existing ones.
In this study, we aimed to assess and disentangle the direct and indirect effects of local temperature on bird abundance. We focused on breeding birds in the European Alps, along a wide elevational gradient (1,300–2,700 m asl) including montane and subalpine forests, the treeline habitat and alpine grasslands and rocky areas at the highest elevation. To our best knowledge, there are no previous studies aimed at quantitatively disentangling direct and indirect effects of local climate on species distribution/abundance encompassing such a wide elevational gradient. We expected a clear indirect effect of local temperature in all bird species: across such a wide elevational gradient, with forest gradually transitioning into alpine grassland, a strong effect of local climate on vegetation should be supposed, as climate is the main determinant of vegetation characteristics, and a definitive effect of vegetation on birds can be expected due to the striking differences in vegetation structure and composition along the gradient. Concerning the direct effect of local temperature, we expected strong interspecific differences, rather than generalized patterns, as a consequence of species-specific thermal niches and different degree of overlap with the climatic conditions of the study area. More specifically, we expected mountain generalist species to be more associated with warmer sites, given the harsh climate of our inner-Alpine study area (Adler et al., 2015). Conversely, for mountain specialists (e.g., water pipit Anthus spinoletta), we expected a preference for relatively cold microclimates (Chamberlain et al., 2013; Brambilla et al., 2016, 2017; mountain specialist vs generalists were distinguished following Lehikoinen et al., 2019).
Materials and Methods
Data collection
Data were collected as previously described in Ceresa et al. (2020a). Specifically, fieldwork took place in the central-eastern Italian Alps (Wipptal, South Tyrol), immediately south of the main Alpine watershed, within a 3,400 ha-wide area (46.96° N, 11.50° E). This area spans from approximately 1,300 m asl (just above the bottom valley) to more than 2,700 m asl at the highest peaks. At lower elevation, mountainsides are mostly covered by woodlands, dominated by spruce Picea abies and European larch Larix decidua. Above the timberline (approximately 2,000 m asl, but highly variable), wide areas are covered by bushes (mainly Rhododendron spp.) and scattered larches, whereas alpine grasslands, rocks and scree slopes characterize the upper elevation belt.
Birds were surveyed during the breeding season of most local breeding species (May–July) in 2018, by means of point counts (N = 109, Fig. 1) carried out by expert ornithologists. The rugged topography, and the consequent low accessibility of some areas, made a random selection of the sampling points unfeasible. For this reason, we carried out the point counts along accessible transects, often along footpaths; we chose the location of each point based on a minimum distance of 200 m to the previous one, in order to avoid potential replicated counts of the same individuals (Chamberlain et al., 2016).
Figure 1. Study area.
Distribution of the sampling points in the study area (central-eastern European Alps, Italy).
At each sampling point, the surveyor recorded all birds observed or heard during 10 min within a 100 m radius. All points were visited three times (survey sessions: 30 May–6 June, 19–23 June and 13–18 July). The survey timing allowed excluding the main migration periods of locally breeding species. Counts were carried out in the morning, between 5:30 and 11:30, and avoiding poor weather conditions (i.e., rain or strong wind).
Vegetation and other land cover characteristics within a 100 m radius were recorded at each sampling point by the surveyor, with the aid of detailed aerial photos (as described in Ceresa et al. (2020a)). These variables included the percentage cover of tree canopy (i.e., vegetation higher than 2 m), bushes (woody vegetation lower than 2 m, mostly represented by Rhododendron shrublands), grassland (areas with no canopy and covered by grassland vegetation) and rocks/scree (emerging bedrock or scree-covered patches). The percentage cover of each variable was thus visually estimated in the field to the nearest 5%. Variables covering less than 5% of the plot surface (as defined by the 100 m radius) were assigned a 1% cover value. We also recorded information about woodland composition, by visually estimating the proportion of tree canopy cover occupied by each arboreal species (excluding rare species, i.e., representing less than 20% of the tree canopy cover).
In order to obtain climatic information at the site level, at each sampling point we placed an iButton data logger (models DS1921G and DS1922L; Maxim Integrated, San Jose, CA, USA), which recorded temperatures hourly during the entire study period. Loggers were placed at the ground level and were protected from the direct solar radiation by means of a white plastic panel. Further details about loggers’ placement and use, as well as the temperature trends recorded at each point during the study period, are provided in Ceresa et al. (2020a). As we collected such fine-scale temperature values, we did not consider topographic variables in our study. In fact, the effects of topography on bird abundance are very likely to be largely indirect, through a strong influence on microclimate (especially temperature) and, consequently, also on vegetation. Our fine-scale temperatures were indeed clearly related to both elevation and aspect, as expected (see Fig. S1). Relevant direct effects of topography on bird abundance (non-mediated by local temperature and vegetation) are indeed hardly to occur, especially for elevation and aspect.
Statistical analysis
We used structural equation modelling to disentangle the direct effect of microclimate on bird abundance and the indirect effects of local temperature, mediated by vegetation characteristics. Structural equation modelling consists in a multivariate linear regression analysis, which allows assessing the effect of a predictor on a dependent variable through mediating variables. Therefore, following this approach, it is possible to model simultaneously both direct and indirect effect of a predictor on a dependent variable (Rosseel, 2012). In the model structure we adopted, variables describing vegetation characteristics represented the mediating variables in the indirect pathways relating local temperature and bird abundance (see the path diagrams in Fig. 2). Models were fitted using the sem function of package lavaan version 0.6-7 (Rosseel, 2012) in program R version 3.5.3 (R Core Team, 2019); we used the Satorra-Bentler maximum likelihood test statistics, which provides model fit measures that are robust to data non-normality (Satorra & Bentler, 1994). We considered standardized path coefficient as significant at p ≤ 0.1, to reduce the risk of type II error associated with the adopted statistical approach (Shipley, 2016). An indirect effect was considered as significant when all single path coefficients of the indirect pathway were significant at p ≤ 0.1. We compared direct and indirect effects of local temperature by comparing the standardized coefficients of direct and indirect pathways.
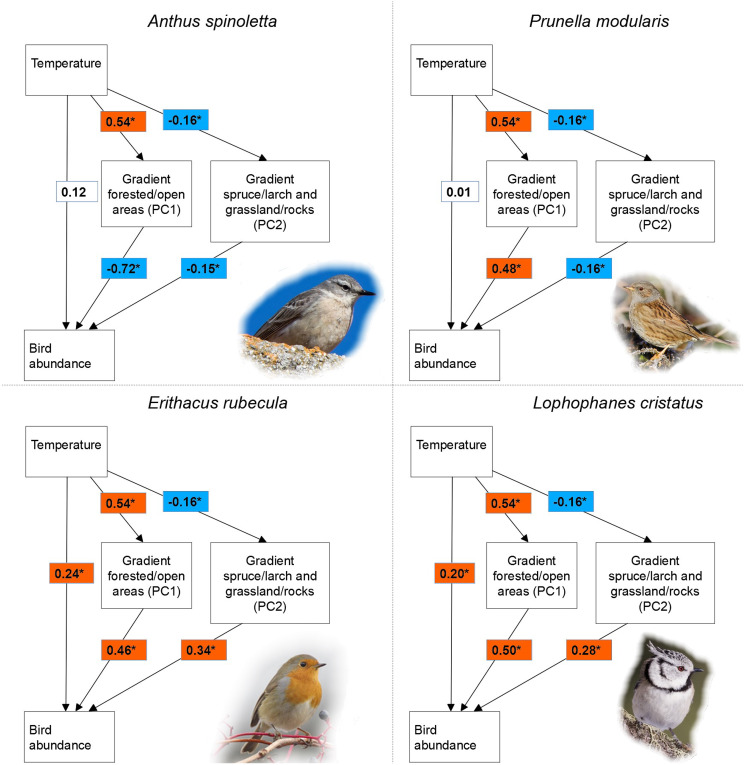
Figure 2. Relationship among temperature, vegetation characteristics and bird abundance.
Relationship among temperature, vegetation characteristics (PC1 and PC2) and bird abundance according to structural equation modelling in four of 15 investigated bird species breeding in a mountain area. The reported values are standardized regression coefficients and the asterisk indicate a significant effect (p ≤ 0.1).
To describe vegetation characteristics in our models, we used two principal components describing overall 60% of the variability in the vegetation data collected in the field (PC1 = 40%, PC2 = 20%). Principal component analysis was carried out using the prcomp function in the base R package. The first component was positively associated with forested areas and negatively with open areas (grassland and rocks), while the second component described a gradient in woodland composition (it was negatively associated with larch, one of the two dominant arboreal species), as well as the degree of grassland cover in open areas (positive association with rocks, negative with grassland; see Table 1). As local climatic predictor, we used the mean temperature calculated for each sampling point across the entire study period, because it allows describing the general climatic conditions at a location (Virkkala et al., 2008; Stralberg et al., 2009). Mean temperatures during the study period varied across the study area between 6.6 °C and 14.8 °C, and obviously showed a clear trend along the elevation gradient (Fig. S1). As a measure of bird abundance, for each species we used the maximum number of individuals detected at each sampling point during the three sampling sessions. Unlike other studies dealing with mountain species’ ecology, but not aimed at quantitative disentangling direct and indirect climate effects (e.g., Chamberlain et al., 2013; Ceresa et al., 2020a), we considered the points recorded over the complete elevational gradient for all species. This implied that, for each species, we included also areas with clearly unsuitable vegetation characteristics. Given that vegetation characteristics are largely determined by local climate, the selection of sub-samples of the sampling points based on vegetation characteristics (as frequently done in other studies with different aims), would have biased the estimation of the indirect temperature effects on bird abundance (likely leading to strong underestimation of such effects). We only considered those species detected at least once at a minimum of 20 sampling points, i.e., 15 species. These species (listed in Table 2) show a variety of different habitat preferences and specialization, as well as different nesting habits and migration strategies, therefore our results are based on a highly representative species set.
Table 1. Relationship between vegetation variables collected in the field and PCA components used to descibe vegetation.
Relationship between the two principal components (used to describe vegetation structure and composition in structural equation models) and each land cover category.
| Land cover category | PC1 | PC2 |
|---|---|---|
| Bushes (% cover) | 0.053 | −0.663 |
| Rocks/scree (% cover) | −0.328 | 0.424 |
| Tree canopy (% cover) | 0.572 | 0.340 |
| Undergrowth (% cover) | 0.318 | −0.163 |
| Grassland (% cover) | −0.493 | −0.368 |
| Larches (% of total canopy cover) | 0.468 | −0.321 |
Table 2. Direct and indirect effects of local climate on bird abundance.
Effects of vegetation (PC1 and PC2) and temperature on breeding bird abundance according to structural equation modelling (p-values in parentheses), and variance in bird abundance explained (R2) for each species. Effects are reported as standardized coefficients. Only significant effects are reported (p ≤ 0.1). An indirect effect is considered significant when all path coefficients of the indirect effect path are significant at p ≤ 0.1. Model structure is shown in Fig. 2.
| Species | PC1 | PC2 | Temperature (direct effect) | Indirect temperature effect through PC1 | Indirect temperature effect through PC2 | R2 |
|---|---|---|---|---|---|---|
| Anthus spinoletta | −0.716 (0.000) | −0.145 (0.040) | −0.387 (0.000) | 0.023 (0.191) | 0.44 | |
| Certhia familiaris | 0.485 (0.000) | 0.349 (0.000) | 0.262 (0.000) | −0.055 (0.080) | 0.37 | |
| Erithacus rubecula | 0.460 (0.000) | 0.341 (0.001) | 0.244 (0.002) | 0.249 (0.000) | −0.054 (0.098) | 0.46 |
| Fringilla coelebs | 0.766 (0.000) | 0.414 (0.000) | 0.63 | |||
| Lophophanes cristatus | 0.499 (0.000) | 0.283 (0.005) | 0.203 (0.025) | 0.270 (0.000) | −0.045 (0.177) | 0.44 |
| Oenanthe oenanthe | −0.298 (0.001) | −0.161 (0.003) | 0.19 | |||
| Periparus ater | 0.704 (0.000) | 0.291 (0.000) | 0.162 (0.008) | 0.381 (0.000) | −0.046 (0.086) | 0.68 |
| Phylloscopus collybita | 0.328 (0.005) | 0.178 (0.006) | 0.17 | |||
| Poecile montanus | 0.451 (0.000) | −0.265 (0.004) | 0.244 (0.000) | 0.042 (0.095) | 0.29 | |
| Prunella modularis | 0.482 (0.000) | −0.164 (0.051) | 0.261 (0.000) | 0.026 (0.167) | 0.34 | |
| Pyrrhula pyrrhula | 0.440 (0.000) | 0.267 (0.000) | 0.132 (0.069) | 0.238 (0.000) | −0.042 (0.088) | 0.31 |
| Regulus regulus | 0.301 (0.000) | 0.449 (0.000) | 0.289 (0.002) | 0.163 (0.000) | −0.029 (0.071) | 0.41 |
| Sylvia atricapilla | 0.309 (0.005) | 0.187 (0.096) | 0.167 (0.004) | 0.21 | ||
| Turdus torquatus | −0.332 (0.007) | 0.053 (0.123) | 0.13 | |||
| Turdus viscivorus | 0.199 (0.058) | 0.186 (0.029) | 0.107 (0.060) | 0.11 |
We evaluated model goodness-of-fit by a chi-square fit statistic, which indicates a significant lack of fit when p < 0.05 (Hooper, Coughlan & Mullen, 2008). We also used other fit metrics commonly adopted in structural equation modelling: Comparative Fit Index (CFI), Tucker-Lewis Index (TLI), root mean square error of approximation (RMSEA) and standardized root mean square residual (SRMR). A good model fit is indicated by CFI and TLI > 0.95, RMSEA < 0.07 and SRMR < 0.05 (Hooper, Coughlan & Mullen, 2008). Using and reporting such a variety of fit metrics is recommended in structural equation modelling, because each one reflects a different aspect of model fit (Crowley & Fan, 1997; Hooper, Coughlan & Mullen, 2008). In order to test for spatial autocorrelation, we calculated Moran’s I for model residuals using ArcGis 10.8 (ESRI, Redlands, CA, USA). Moran’s I values > 0.3 are considered as relatively large (Lichstein et al., 2002).
Results
For all species, structural equation models indicated a significant effect of vegetation characteristics on bird abundance (Table 2). Out of 15 species, PC1 had a significant effect in 14 and PC2 in 10 species. Given the significant effect of temperature on both PC1 (β = 0.54, p < 0.001) and PC2 (β = −0.16, p = 0.063), indirect pathways were always significant in case of significant effect of a principal component on bird abundance. Indirect temperature effects were indeed found in all species, while direct temperature effects occurred in seven species (Table 2). When present, direct temperature effects were always positive and showed a highly variable relative importance compared to indirect effects (Table 2). Variance in bird abundance explained by structural equation models ranged between 11% and 68%, with only four species below 20% (Table 2). For all species, models fitted the data well according to the chi-square test (χ2 = 1.265, df = 1, p = 0.261) and all the other fit metrics (CFI and TLI > 0.95, RMSEA < 0.07 and SRMR < 0.05). For seven species, Moran’s I test indicated the lack of spatial autocorrelation (Moran’s I range: −0.037 to 0.065; p > 0.05; see Table S1). In the other species the test was significant, but with very low or low Moran’s I values (approx. 0.1–0.2; Table S1), indicating a weak clustering that is very unlikely to affect results. The only exception is represented by the common chiffchaff Phylloscopus collybita, which showed a relatively high spatial autocorrelation (Moran’s I = 0.414); the results for this species should be cautiously considered.
Discussion
Disentangling direct and indirect effects of climate on species occurrence and abundance is crucial to assess distribution and population drivers, and to evaluate the potential impacts of climate change on wild species (de Chazal & Rounsevell, 2009). The correlative link between climate and species (especially for homoeotherm animals) often reflects an indirect effect, which is actually mediated by other factors associated with climate, such as habitat characteristics or the availability of key resources (Brambilla et al., 2019a). Here, we investigated the drivers of bird abundance in a mountain context, where climate and habitat effects are intermingled, explicitly distinguishing between direct and vegetation-mediated impacts of local temperatures.
As expected, we observed a significant indirect effect of local temperature via vegetation characteristics on the abundance of all the species we considered. This suggests that such a pattern could be generalized, or at least very common in bird communities of temperate mountain regions, given the wide ecological spectrum represented in our species set. Temperatures at sampling sites had a significant effect on both principal components describing vegetation, with a stronger influence on the most important one (PC1), which accounts for the difference between forested and open areas (Fig. 2). This is not surprising, as climatic factors are crucial in determining the treeline position, jointly with several additional factors such as human land use and geomorphology (Körner & Paulsen, 2004; Chauchard et al., 2010; Leonelli et al., 2011). The observed effects of the two vegetation principal components on bird abundance are fully consistent with the known habitat preferences of each species (e.g., Brichetti & Fracasso, 2007, 2008; Chamberlain et al., 2016). Unlike indirect effects, direct temperature effects only occurred in some species, consistently with our expectation of strong interspecific differences and lack of generalized patterns.
Direct effects of local climate for birds in the Alps
All the seven species showing significant direct temperature effects (see Table 2) are woodland or shrubland birds and are not mountain specialists. The distribution of some of these species in Italy widely overlaps with the main mountain chains, but they cannot be considered as mountain specialists because they breed also in hilly and low elevation areas, and in nearby countries they are also widely distributed across lowlands (coal tit Periparus ater, crested tit Lophophanes cristatus, Eurasian bullfinch Pyrrhula pyrrhula, goldcrest Regulus regulus, mistle thrush Turdus viscivorus; species distributions are available in BirdLife International (2021)). Given the positive direct temperature effect on their abundance, at least part of the woodland within our study area is likely colder than the thermal optimum of these species; our inner-Alpine study area is indeed characterized by an especially severe climate, with long-lasting snow cover (Adler et al., 2015). Such temperature effect could be emphasized by the early breeding of these species: warmer sites likely provide better conditions early in the reproduction season, especially for what concerns the availability of key poikilothermic prey (invertebrates). Two other species showing a positive direct temperature effect (Eurasian blackcap Sylvia atricapilla and European robin Erithacus rubecula) are generalist species with very broad habitat niche, and are very common at low and middle elevations; our study area is probably located towards the coldest extreme of their thermal niche.
In the two open habitat species for which we obtain a sufficient sample for the analysis (water pipit and northern wheatear Oenanthe oenanthe), we found a significant negative temperature effect on abundance, but exclusively indirectly via vegetation. This suggests that, at least in inner Alpine areas like our study site, maintaining and correctly managing high-elevation pastures and grasslands would probably represent effective conservation measures for these species, as proposed also in other studies (e.g., Chamberlain et al., 2013; Brambilla et al., 2018, 2020). Differently from what we expected, our results indeed did not show a direct temperature effect for the water pipit, a mountain-specialist grassland bird that had been reported to be associated with cold climates in other studies focusing on species occurrence (e.g., Brambilla et al., 2017, 2019a), and to experience variation in breeding success according to nest-site orientation (Rauter, Reyer & Bollmann, 2002). The most likely explanation for such differences is that, in this cold, inner-Alpine area, the climatic conditions of alpine grasslands are close to the thermal optimum of this species. On the other side, the previous studies reporting an effect of local climate on this species’ occurrence were carried out at a much larger scale, including also low-elevation, warmer massifs (Brambilla et al., 2017), or took place in more southern and definitely warmer mountain chains (i.e., Apennines; Brambilla et al., 2019a, Brambilla et al., 2020). The large differences in temperature ranges between our and those previously investigated study areas probably explains the differences with our results, which revealed no direct effect of temperature on abundance (besides the different focus on occurrence vs. local abundance, and the different statistical approach). This highlights the importance of spatial scale when studying the influence of climatic factors on species distribution and abundance, as different or even contrasting results may arise from analyses carried out at different scales (e.g., Franklin et al., 2013; Brambilla et al., 2019a).
While the proportion of variance in bird abundance explained by our models was good for most species (see Table 2), the proportion of unexplained variance suggests the more or less important effect of additional factors not considered in this study, such as spatial variation in predation intensity and other biotic interactions, microhabitat characteristics, and possibly also terrain slope in some species (see, e.g., Thompson, 2007; Sherry et al., 2015; Freeman & Montgomery, 2016; Brambilla et al., 2019a). We could not consider also the possible effect of precipitations on bird abundance, because we did not collect precipitation data in the field. However, over such a restricted study area the spatial variation of precipitation is likely to be affected almost only by the reduction of temperatures with elevation (through orographic effect), which should be strictly related to our fine-scale temperature data. Therefore, precipitation data would likely add only limited information to our models.
Implications for research and conservation
Our results are largely consistent with those reported by Duclos, DeLuca & King (2019), who investigated a set of forest bird species breeding in a North American mountain area and found an indirect effect of climate via vegetation in all species, while directs effects occurred only in part of the species and strongly varied in their magnitude. This suggests that such climate-species relationship patterns may be widespread in mountain bird communities, at least in temperate regions and in areas where the number of high-elevation specialists is often very low, as in our study system. This should be taken into account when investigating and predicting future bird distribution and abundance. This could be particularly relevant for generalist mountain birds, as in many cases they may be largely tracking suitable habitat, rather than thermal niches, as our results suggest for several species inhabiting different habitats and different elevation belts. Consequently, accounting also for future changes in vegetation structure and composition would likely allow more reliable predictions than using climatic factors only; this is a complex task, as both human land use and climatic factors should be considered to forecast vegetation changes. In addition, vegetation changes may lag largely behind climate change (Iverson, Prasad & Matthews, 2008; Stralberg et al., 2015): in e.g., the northeastern United States tree species shift has been forecasted to occur in some centuries (Wang et al., 2016). Such a complex and context-dependent scenario (species-specific direct and indirect climate effects, influence of human land use) may help to explain why elevational range shifts of mountain bird distribution assessed until now are poorly consistent across different studies in both magnitude and direction (i.e., uphill/downhill; see Scridel et al. (2018)), in spite of the global scale temperature increase.
While indirect climate change effects via vegetation may be strongly delayed or influenced by human activities, direct effects are likely to impact bird population rapidly, by affecting, e.g., breeding phenology, prey availability, survival and breeding success (e.g., Rodriguez & Bustamante, 2003; Dunn, 2004; Wesołowski et al., 2016; Bison et al., 2020; Ceresa et al., 2020b; Strinella et al., 2020). According to our results, at least in the innermost part of the European Alps the ongoing temperature increase may favour some generalist woodland and shrubland bird species (as suggested, e.g., by Solonen, 2005; Scridel et al., 2017). This could occur due to wider woodland areas with adequate local climatic conditions for these species, but also because of a possibly longer breeding season (Bison et al., 2020). According to Ceresa et al. (2020a), in our study area European robins select warmer areas for breeding during the first part of the reproductive season, thus higher spring temperatures would likely allow start breeding earlier across wider areas, with a consequently larger time window available for subsequent reproduction attempts.
The indirect effect of local climate we found in the two open habitat species here considered highlights the importance of conservation and management of Alpine grasslands for those species. Uphill treeline shift and shrubland expansion have been recorded in several mountain ranges, including the European Alps, and besides temperature increase they are often strongly promoted by land abandonment (Gehrig-Fasel, Guisan & Zimmermann, 2007; Myers-Smith et al., 2011). Therefore, habitat loss for these species needs to be counteracted by maintaining cattle grazing in mountain pastures, managed in an extensive way to avoid overgrazing, which leads to grassland and soil degradation (Garcia-Pausas et al., 2017) and can be detrimental to open-habitat bird species (Brambilla et al., 2020). In addition, the construction of new touristic infrastructures such as ski-pistes should be limited, because they negatively affect high-elevation grassland bird communities (Rolando et al., 2007; Caprio et al., 2011) and are predicted to increasingly overlap with the distribution of high-elevation specialist birds as a consequence of climate change (Brambilla et al., 2016). Other bird species connected to high-elevation open areas could be affected by climatic factors also directly, e.g., the white-winged snowfinch Montifringilla nivalis (Brambilla et al., 2019b), and unfortunately we did not obtain a sufficient sample for this species and other locally breeding high-elevation specialists (alpine accentor Prunella collaris, alpine chough Pyrrhocorax graculus). However, also in case of direct climate effects the aforementioned conservation measures would be recommendable, in order to try buffering against climate change. Given the high terrain complexity of mountain ranges, across large areas of suitable habitat some sites may maintain adequate microclimatic conditions for cold-associated species, possibly acting as local ‘refugia’ despite temperature warming at larger scale (Morelli et al., 2016). Therefore, avoiding habitat loss and degradation as previously described could increase the future amount of refugia maintaining both vegetation and microclimatic suitable characteristics. Furthermore, correctly managed cattle grazing could provide/maintain micro-habitats suitable for foraging for species potentially vulnerable to earlier snowmelt (Brambilla et al., 2018).
Conclusions
The impact of climate and land-use/land-cover changes are among the major threats to biodiversity worldwide, and will exacerbate in the future decades. Properly addressing the nature of their effects on wild species is key to understanding impacts and developing conservation strategies. In this study, considering a set of bird species along a broad elevational gradient, we found a generalised indirect effect via vegetation of local temperatures on birds abundance, while direct effects were less common and were found in some mountain generalist birds. Our work provides an example of disentangling causes and effects when dealing with the combined impact of habitat and local climate on target organisms; a similar framework may be used to address effects and impacts on many other ecosystems, promoting a deeper understanding of species’ response to habitat and climate and the relative changes.
Supplemental Information
Bird abundance, vegetation and mean temperature at each sampling point.
Elevation and aspect jointly explain 56% of variation in mean temperatures according to multiple linear regression (mean temperature ~ elevation + aspect), and both show a significant effect (elevation: β = −1.3, p < 0.0000; aspect: β = −0.3, p = 0.007).
Spatial autocorrelation in model residuals for each species, according to Moran’s I test.
Acknowledgments
We thank Prof. Emilio Barba (Institute Cavanilles of Biodiversity and Evolutionary Biology-University of Valencia) for providing part of the temperature loggers. We are very grateful to D. Chamberlain and an anonymous reviewer for helpful comments on a first draft of the manuscript.
Funding Statement
The present study has been financed by the Research fund of the Museums of South Tyrol, within the project ‘The distribution and conservation status of birds in South Tyrol’, CUP H53C17000260005. The Department of Innovation, Research and University of the Autonomous Province of Bozen/Bolzano covered the Open Access publication costs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Francesco Ceresa conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Petra Kranebitter conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Juan S. Monrós conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Franco Rizzolli conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Mattia Brambilla conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The complete data used in structural equation models, i.e., bird abundancies, mean temperatures and vegetation (as PCA components) for each sampling point is available in the Supplemental File.
References
- Adler et al. (2015).Adler S, Chimani B, Drechsel S, Haslinger K, Hiebl J, Meyer V, Resch G, Rudolph J, Vergeiner J, Zingerle C, Marigo G, Fischer A, Seiser B. Il clima del Tirolo - Alto Adige – Bellunese. Padova: Zentralanstalt fur Meteorologie und Geodynamik, Ripartizione Protezione antincendi e civile – Provincia Autonoma di Bolzano, Agenzia Regionale per la Prevenzione e Protezione Ambientale del Veneto; 2015. [Google Scholar]
- Barnagaud et al. (2011).Barnagaud JY, Crochet PA, Magnani Y, Bernard Laurent A, Menoni E, Novoa C, Gimenez O. Short-term response to the North Atlantic Oscillation but no long-term effects of climate change on the reproductive success of an alpine bird. Journal of Ornithology. 2011;152(3):631–641. doi: 10.1007/s10336-010-0623-8. [DOI] [Google Scholar]
- BirdLife International (2021).BirdLife International IUCN red list for birds. 2021. http://www.birdlife.org. [15 February 2021]. http://www.birdlife.org
- Bison et al. (2020).Bison M, Yoccoz NG, Carlson B, Klein G, Laigle I, Van Reeth C, Asse D, Delestrade A. Best environmental predictors of breeding phenology differ with elevation in a common woodland bird species. Ecology and Evolution. 2020;10(18):10219–10229. doi: 10.1002/ece3.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle & Martin (2015).Boyle WA, Martin K. The conservation value of high elevation habitats to North American migrant birds. Biological Conservation. 2015;192:461–476. doi: 10.1016/j.biocon.2015.10.008. [DOI] [Google Scholar]
- Brambilla et al. (2017).Brambilla M, Caprio E, Assandri G, Scridel D, Bassi E, Bionda R, Celada C, Falco R, Bogliani G, Pedrini P, Rolando A, Chamberlain D. A spatially explicit definition of conservation priorities according to population resistance and resilience, species importance and level of threat in a changing climate. Diversity and Distributions. 2017;23(7):727–738. doi: 10.1111/ddi.12572. [DOI] [Google Scholar]
- Brambilla et al. (2019a).Brambilla M, Gustin M, Cento M, Ilahiane L, Celada C. Predicted effects of climate factors on mountain species are not uniform over different spatial scales. Journal of Avian Biology. 2019a;50(9):577. doi: 10.1111/jav.02162. [DOI] [Google Scholar]
- Brambilla et al. (2020).Brambilla M, Gustin M, Cento M, Ilahiane L, Celada C. Habitat, climate, topography and management differently affect occurrence in declining avian species: implications for conservation in changing environments. Science of the Total Environment. 2020;742:140663. doi: 10.1016/j.scitotenv.2020.140663. [DOI] [PubMed] [Google Scholar]
- Brambilla et al. (2016).Brambilla M, Pedrini P, Rolando A, Chamberlain DE. Climate change will increase the potential conflict between skiing and high-elevation bird species in the Alps. Journal of Biogeography. 2016;43(11):2299–2309. doi: 10.1111/jbi.12796. [DOI] [Google Scholar]
- Brambilla et al. (2018).Brambilla M, Resano-Mayor J, Scridel D, Anderle M, Bogliani G, Braunisch V, Capelli F, Cortesi M, Horrenberger N, Pedrini P, Sangalli B, Chamberlain D, Arlettaz R, Rubolini D. Past and future impact of climate change on foraging habitat suitability in a high-alpine bird species: management options to buffer against global warming effects. Biological Conservation. 2018;221:209–218. doi: 10.1016/j.biocon.2018.03.008. [DOI] [Google Scholar]
- Brambilla et al. (2019b).Brambilla M, Scridel D, Sangalli B, Capelli F, Pedrini P, Bogliani G, Rubolini D. Ecological factors affecting foraging behaviour during nestling rearing in a high-elevation species, the White-winged Snowfinch (Montifringilla nivalis) Ornis Fenn. 2019b;96:142–151. [Google Scholar]
- Brichetti & Fracasso (2007).Brichetti P, Fracasso G. Ornitologia italiana, Vol. 4 – Apodidae-Prunellidae. Wales: Oasi Alberto Perdisa Editore; 2007. [Google Scholar]
- Brichetti & Fracasso (2008).Brichetti P, Fracasso G. Ornitologia italiana, Vol. 5 – Turdidae-Cisticolidae. Wales: Oasi Alberto Perdisa Editore; 2008. [Google Scholar]
- Cadena et al. (2012).Cadena CD, Kozak KH, Gomez JP, Parra JL, McCain CM, Bowie RCK, Carnaval AC, Moritz C, Rahbek C, Roberts TE, Sanders NJ, Schneider CJ, VanDerWal J, Zamudio KR, Graham CH. Latitude, elevational climatic zonation and speciation in new world vertebrates. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1726):194–201. doi: 10.1098/rspb.2011.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprio et al. (2011).Caprio E, Chamberlain DE, Isaia M, Rolando A. Landscape changes caused by high altitude ski-pistes affect bird species richness and distribution in the Alps. Biological Conservation. 2011;144(12):2958–2967. doi: 10.1016/j.biocon.2011.08.021. [DOI] [Google Scholar]
- Ceresa et al. (2020b).Ceresa F, Belda EJ, Brambilla M, Gómez J, Mompó C, Monrós JS. Factors shaping breeding phenology in birds: an assessment of two sympatric Acrocephalus warblers with different life histories. Ardeola. 2020b;67(2):371–385. doi: 10.13157/arla.67.2.2020.ra9. [DOI] [Google Scholar]
- Ceresa et al. (2020a).Ceresa F, Brambilla M, Rizzolli F, Monrós JS, Kranebitter P. Within-season movements of Alpine songbird distributions are driven by fine-scale environmental characteristics. Scientific Reports. 2020a;10(1):5747. doi: 10.1038/s41598-020-62661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain et al. (2016).Chamberlain DE, Brambilla M, Caprio E, Pedrini P, Rolando A. Alpine bird distributions along elevation gradients: the consistency of climate and habitat effects across geographic regions. Oecologia. 2016;181(4):1139–1150. doi: 10.1007/s00442-016-3637-y. [DOI] [PubMed] [Google Scholar]
- Chamberlain et al. (2013).Chamberlain DE, Negro M, Caprio E, Rolando A. Assessing the sensitivity of alpine birds to potential future changes in habitat and climate to inform management strategies. Biological Conservation. 2013;167:127–135. doi: 10.1016/j.biocon.2013.07.036. [DOI] [Google Scholar]
- Chauchard et al. (2010).Chauchard S, Beilhe F, Denis N, Carcaillet C. An increase in the upper tree-limit of silver fir (Abies alba Mill.) in the Alps since the mid-20th century: a land-use change phenomenon. Forest Ecology and Management. 2010;259(8):1406–1415. doi: 10.1016/j.foreco.2010.01.009. [DOI] [Google Scholar]
- Cheviron & Brumfield (2012).Cheviron ZA, Brumfield RT. Genomic insights into adaptation to high-altitude environments. Heredity. 2012;108(4):354–361. doi: 10.1038/hdy.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley & Fan (1997).Crowley SL, Fan X. Structural equation modeling: basic concepts and applications in personality assessment research. Journal of Personality Assessment. 1997;68(3):508–531. doi: 10.1207/s15327752jpa6803_4. [DOI] [PubMed] [Google Scholar]
- de Chazal & Rounsevell (2009).de Chazal J, Rounsevell MDA. Land-use and climate change within assessments of biodiversity change: a review. Global Environmental Change. 2009;19(2):306–315. doi: 10.1016/j.gloenvcha.2008.09.007. [DOI] [Google Scholar]
- De Gabriel Hernando et al. (2021).De Gabriel Hernando M, Fernández-Gil J, Roa I, Juan J, Ortega F, de la Calzada F, Revilla E. Warming threatens habitat suitability and breeding occupancy of rear-edge alpine bird specialists. Ecography. 2021;44(8):1191–1204. doi: 10.1111/ecog.05593. [DOI] [Google Scholar]
- Dirnböck, Essl & Rabitsch (2011).Dirnböck T, Essl F, Rabitsch W. Disproportional risk for habitat loss of high altitude endemic species under climate change. Global Change Biology. 2011;17(2):990–996. doi: 10.1111/j.1365-2486.2010.02266.x. [DOI] [Google Scholar]
- Duclos, DeLuca & King (2019).Duclos TR, DeLuca WV, King DI. Direct and indirect effects of microclimate on bird abundance along elevation gradients in the Northern Appalachian mountains. Diversity and Distributions. 2019;25(11):1670–1683. doi: 10.1111/ddi.12968. [DOI] [Google Scholar]
- Dunn (2004).Dunn P. Breeding dates and reproductive performance. Integrative Ecology: From Molecules to Ecosystems. 2004;35:69–87. doi: 10.1016/S0065-2504(04)35004-X. [DOI] [Google Scholar]
- Elsen et al. (2017).Elsen PR, Tingley MW, Kalyanaraman R, Ramesh K, Wilcove DS. The role of competition, ecotones, and temperature in the elevational distribution of Himalayan birds. Ecology. 2017;98(2):337–348. doi: 10.1002/ecy.1669. [DOI] [PubMed] [Google Scholar]
- Engler et al. (2017).Engler JO, Stiels D, Schidelko K, Strubbe D, Quillfeldt P, Brambilla M. Avian SDMs: current state, challenges, and opportunities. Journal of Avian Biology. 2017;48(12):1483–1504. doi: 10.1111/jav.01248. [DOI] [Google Scholar]
- Franklin et al. (2013).Franklin J, Davis FW, Ikegami M, Syphard AD, Flint LE, Flint AL, Hannah L. Modeling plant species distributions under future climates: how fine scale do climate projections need to be? Global Change Biology. 2013;19(2):473–483. doi: 10.1111/gcb.12051. [DOI] [PubMed] [Google Scholar]
- Freeman & Montgomery (2016).Freeman BG, Montgomery G. Interspecific aggression by the Swainson’s thrush (Catharus ustulatus) may limit the distribution of the threatened Bicknell’s thrush (Catharus bicknelli) in the Adirondack Mountains. The Condor. 2016;118(1):169–178. doi: 10.1650/CONDOR-15-145.1. [DOI] [Google Scholar]
- Frey, Hadley & Betts (2016).Frey SJ, Hadley AS, Betts MG. Microclimate predicts within-season distribution dynamics of montane forest birds. Diversity and Distributions. 2016;22(9):944–959. doi: 10.1111/ddi.12456. [DOI] [Google Scholar]
- Garcia-Pausas et al. (2017).Garcia-Pausas J, Romanyà J, Montané F, Rios AI, Taull M, Rovira P, Casals P. Are soil carbon stocks in mountain grasslands compromised by land-use changes?. High Mountain Conservation in a Changing World; Berlin: Springer; 2017. pp. 207–230. [Google Scholar]
- Gehrig-Fasel, Guisan & Zimmermann (2007).Gehrig-Fasel J, Guisan A, Zimmermann NE. Tree line shifts in the Swiss Alps: climate change or land abandonment? Journal of Vegetation Science. 2007;18(4):571–582. doi: 10.1111/j.1654-1103.2007.tb02571.x. [DOI] [Google Scholar]
- Goodenough & Hart (2013).Goodenough AE, Hart AG. Correlates of vulnerability to climate-induced distribution changes in European avifauna: habitat, migration and endemism. Climatic Change. 2013;118(3–4):659–669. doi: 10.1007/s10584-012-0688-x. [DOI] [Google Scholar]
- Hooper, Coughlan & Mullen (2008).Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Electronic Journal of Business Research Methods. 2008;6:53–60. doi: 10.21427/D7CF7R. [DOI] [Google Scholar]
- Iverson, Prasad & Matthews (2008).Iverson L, Prasad A, Matthews S. Modeling potential climate change impacts on the trees of the Northeastern United States. Mitigation and Adaptation Strategies for Global Change. 2008;13(5–6):487–516. doi: 10.1007/s11027-007-9129-y. [DOI] [Google Scholar]
- Jongsomjit et al. (2013).Jongsomjit D, Stralberg D, Gardali T, Salas L, Wiens J. Between a rock and a hard place: the impacts of climate change and housing development on breeding birds in California. Landscape Ecology. 2013;28(2):187–200. doi: 10.1007/s10980-012-9825-1. [DOI] [Google Scholar]
- Jähnig et al. (2020).Jähnig S, Sander MM, Caprio E, Rosselli D, Rolando A, Chamberlain D. Microclimate affects the distribution of grassland birds, but not forest birds, in an Alpine environment. Journal of Ornithology. 2020;161(3):677–689. doi: 10.1007/s10336-020-01778-5. [DOI] [Google Scholar]
- Körner & Ohsawa (2006).Körner C, Ohsawa M. Mountain systems, ecosystem and human well-being: current state and trends. In: Hassan R, Scholes R, Ash N, editors. Millennium Ecosystem Assessment. Vol. 1. Washington, D.C: Island Press; 2006. pp. 681–716. [Google Scholar]
- Körner & Paulsen (2004).Körner C, Paulsen J. A world-wide study of high altitude treeline temperatures. Journal of Biogeography. 2004;31(5):713–732. doi: 10.1111/j.1365-2699.2003.01043.x. [DOI] [Google Scholar]
- Leech & Crick (2007).Leech D, Crick HQP. Influence of climate change on the abundance, distribution and phenology of woodland bird species in temperate regions. Ibis. 2007;149(Suppl. 1):128–145. doi: 10.1111/j.1474-919X.2007.00729.x. [DOI] [Google Scholar]
- Lehikoinen et al. (2019).Lehikoinen A, Brotons L, Calladine J, Campedelli T, Escandell V, Flousek J, Grueneberg C, Haas F, Harris S, Herrando S, Magne Husby M, Jiguet F, Kålås JA, Åke Lindström Å, Lorrillière R, Molina B, Pladevall C, Calvi G, Sattler T, Schmid H, Sirkiä1 PM, Teufelbauer N, Trautmann S. Declining population trends of European mountain birds. Global Change Biology. 2019;25(2):577–588. doi: 10.1111/gcb.14522. [DOI] [PubMed] [Google Scholar]
- Leonelli et al. (2011).Leonelli G, Pelfini M, di Cella UM, Garavaglia V. Climate warming and the recent treeline shift in the European Alps: the role of geomorphological factors in high-altitude sites. AMBIO. 2011;40(3):264–273. doi: 10.1007/s13280-010-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein et al. (2002).Lichstein JW, Simons TR, Shriner SA, Franzreb KE. Spatial autocorrelation and autoregressive models in ecology. Ecological Monographs. 2002;72:445–463. doi: 10.1890/0012-9615(2002)072[0445:SAAAMI]2.0.CO;2. [DOI] [Google Scholar]
- Mantyka-Pringle, Martin & Rhodes (2012).Mantyka-Pringle CS, Martin TG, Rhodes JR. Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Global Change Biology. 2012;18(4):1239–1252. doi: 10.1111/j.1365-2486.2011.02593.x. [DOI] [Google Scholar]
- Martin (2001).Martin TE. Abiotic vs. biotic influences on habitat selection of coexisting species: climate change impacts? Ecology. 2001;82:175–188. doi: 10.1890/0012-9658(2001)082. [DOI] [Google Scholar]
- Morelli et al. (2016).Morelli TL, Daly C, Dobrowski SZ, Dulen DM, Ebersole JL, Jackson ST, Lundquist JD, Millar CI, Maher SP, Monahan WP, Nydick KR, Redmond KT, Sawyer SC, Stock S, Beissinger SR. Managing climate change refugia for climate adaptation. PLOS ONE. 2016;11(8):e0159909. doi: 10.1371/journal.pone.0159909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers et al. (2000).Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403(6772):853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Myers-Smith et al. (2011).Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T, Blok D, Tape KD, Macias-Fauria M, Sass-Klaassen U, Levesque E, Boudreau S, Ropars P, Hermanutz L, Trant A, Collier LS, Weijers S, Rozema J, Rayback SS, Schmidt NM, Schaepman-Strub G, Wipf S, Rixen C, Menard CC, Venn S, Goetz S, Andreu-Hayles L, Elmendorf S, Ravolainen V, Welker J, Grogan P, Epstein HE, Hik DS. Shrub expansion in tundra eco-systems: dynamics, impacts and research priorities. Environmental Research Letters. 2011;6(4):045509. doi: 10.1088/1748-9326/6/4/045509. [DOI] [Google Scholar]
- Pearce-Higgins et al. (2015).Pearce-Higgins JW, Eglington SM, Martay B, Chamberlain DE. Drivers of climate change impacts on bird communities. Journal of Animal Ecology. 2015;84(4):943–954. doi: 10.1111/1365-2656.12364. [DOI] [PubMed] [Google Scholar]
- Péron & Altwegg (2015).Péron G, Altwegg R. Twenty-five years of change in southern African passerine diversity: nonclimatic factors of change. Global Change Biology. 2015;21(9):3347–3355. doi: 10.1111/gcb.12909. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019).R Core Team . R: a language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing; 2019. [Google Scholar]
- Rauter, Reyer & Bollmann (2002).Rauter CM, Reyer HU, Bollmann K. Selection through predation, snowfall and microclimate on nest-site preferences in the Water Pipit Anthus spinoletta. Ibis. 2002;144(3):433–444. doi: 10.1046/j.1474-919X.2002.00013.x. [DOI] [Google Scholar]
- Rodriguez & Bustamante (2003).Rodriguez C, Bustamante J. The effect of weather on lesser kestrel breeding success: can climate change explain historical population declines? Journal of Animal Ecology. 2003;72(5):793–810. doi: 10.1046/j.1365-2656.2003.00757.x. [DOI] [Google Scholar]
- Rodríguez et al. (2007).Rodríguez JP, Brotons L, Bustamante J, Seoane J. The application of predictive modelling of species distribution to biodiversity conservation. Diversity and Distributions. 2007;13(3):243–251. doi: 10.1111/j.1472-4642.2007.00356.x. [DOI] [Google Scholar]
- Rolando et al. (2007).Rolando A, Caprio E, Rinaldi E, Ellena I. The impact of high-altitude ski-runs on alpine grassland bird communities. Journal of Applied Ecology. 2007;44(1):210–219. doi: 10.1111/j.1365-2664.2006.01253.x. [DOI] [Google Scholar]
- Rosseel (2012).Rosseel Y. Lavaan: an R package for structural equation modeling and more. Version 0.5-12 (BETA) Journal of Statistical Software. 2012;48(2):1–36. doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- Ruggiero & Hawkins (2008).Ruggiero A, Hawkins BA. Why do mountains support so many species of birds? Ecography. 2008;31(3):306–315. doi: 10.1111/j.0906-7590.2008.05333.x. [DOI] [Google Scholar]
- Sala et al. (2000).Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Satorra & Bentler (1994).Satorra A, Bentler PM. Corrections to test statistics and standard errors in covariance structure analysis. In: von Eye A, Clogg CC, editors. Latent Variables Analysis: Applications for Developmental Research. New York: Sage Publications, Inc; 1994. pp. 399–419. [Google Scholar]
- Scridel et al. (2017).Scridel D, Bogliani G, Pedrini P, Iemma A, von Hardenberg A, Brambilla M. Thermal niche predicts recent changes in range size for bird species. Climate Research. 2017;73:207–216. doi: 10.3354/cr01477. [DOI] [Google Scholar]
- Scridel et al. (2018).Scridel D, Brambilla M, Martin K, Lehikoinen A, Iemma A, Matteo A, Jähnig S, Caprio E, Bogliani G, Pedrini P, Rolando A, Arlettaz R, Chamberlain D. A review and meta-analysis of the effects of climate change on Holarctic mountain and upland bird populations. Ibis. 2018;160:489–515. doi: 10.1111/ibi.12585. [DOI] [Google Scholar]
- Sherry et al. (2015).Sherry TW, Wilson S, Hunter S, Holmes RT. Impacts of nest predators and weather on reproductive success and population limitation in a long-distance migratory songbird. Journal of Avian Biology. 2015;46(6):559–569. doi: 10.1111/jav.00536. [DOI] [Google Scholar]
- Shipley (2016).Shipley B. Cause and correlation in biology: a user’s guide to path analysis, structural equations and causal inference with R. Second Edition. Cambridge: Cambridge University Press; 2016. [Google Scholar]
- Solonen (2005).Solonen T. Breeding of the tawny owl Strix aluco in Finland: responses of a southern colonist to the highly variable environment of the north. Ornis Fennica. 2005;82:97–106. [Google Scholar]
- Stralberg et al. (2015).Stralberg D, Bayne EM, Cumming SG, Sólymos P, Song SJ, Schmiegelow FKA. Conservation of future boreal forest bird communities considering lags in vegetation response to climate change: a modified refugia approach. Diversity and Distributions. 2015;21(9):1112–1128. doi: 10.1111/ddi.12356. [DOI] [Google Scholar]
- Stralberg et al. (2009).Stralberg D, Jongsomjit D, Howell CA, Snyder MA, Alexander JD, Wiens JA, Root TL. Re-shuffling of species with climate disruption: a no-analog future for California birds? PLOS ONE. 2009;4:e6825. doi: 10.1371/journal.pone.0006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strinella et al. (2020).Strinella E, Scridel D, Brambilla M, Schano C, Korner-Nievergelt F. Potential sex-dependent effects of weather on apparent survival of a high-elevation specialist. Scientific Reports. 2020;10(1):1–13. doi: 10.1038/s41598-020-65017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson (2007).Thompson FR. Factors affecting nest predation on forest songbirds in North America. Ibis. 2007;149:98–109. doi: 10.1111/j.1474-919X.2007.00697.x. [DOI] [Google Scholar]
- Virkkala et al. (2008).Virkkala R, Heikkinen RK, Leikola N, Luoto M. Projected large-scale range reductions of northern-boreal land bird species due to climate change. Biological Conservation. 2008;141(5):1343–1353. doi: 10.1016/j.biocon.2008.03.007. [DOI] [Google Scholar]
- Wang et al. (2016).Wang WJ, He HS, Thompson FR, Fraser JS, Dijak WD. Changes in forest biomass and tree species distribution under climate change in the Northeastern United States. Landscape Ecology. 2016;32(7):1399–1413. doi: 10.1007/s10980-016-0429-z. [DOI] [Google Scholar]
- Wesołowski et al. (2016).Wesołowski T, Cholewa M, Hebda G, Maziarz M, Rowiński P. Immense plasticity of timing of breeding in a sedentary forest passerine, Poecile palustris. Journal of Avian Biology. 2016;47(1):129–133. doi: 10.1111/jav.00733. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bird abundance, vegetation and mean temperature at each sampling point.
Elevation and aspect jointly explain 56% of variation in mean temperatures according to multiple linear regression (mean temperature ~ elevation + aspect), and both show a significant effect (elevation: β = −1.3, p < 0.0000; aspect: β = −0.3, p = 0.007).
Spatial autocorrelation in model residuals for each species, according to Moran’s I test.
Data Availability Statement
The following information was supplied regarding data availability:
The complete data used in structural equation models, i.e., bird abundancies, mean temperatures and vegetation (as PCA components) for each sampling point is available in the Supplemental File.