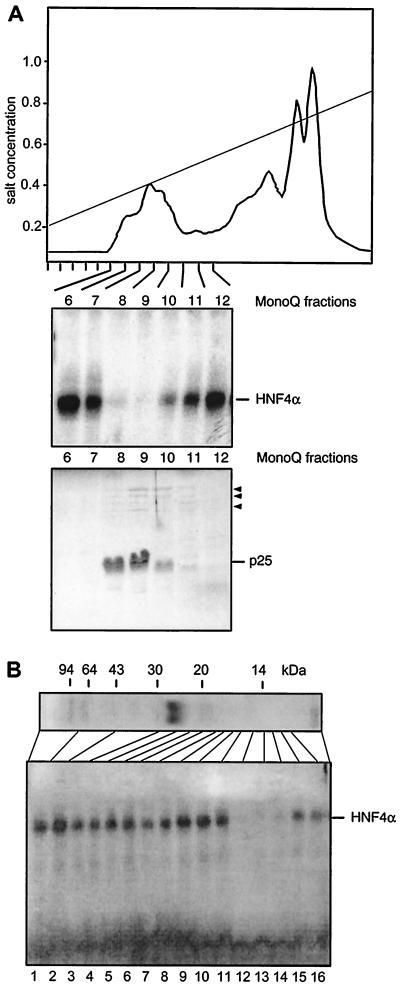
FIG. 2.
Purification and characterization of the HNF4 inhibitor. (A) Elution profile of a heat-treated Xenopus egg extract on a MonoQ column using a salt gradient from 0.2 to 1.0 M NaCl. An aliquot of 0.1 μl of each column fraction was added to a gel retardation assay using rat liver extract and the HNF4 binding site of the Xenopus HNF1α promoter (44). The results are given for fractions 6 to 12 only, as the other fractions did not contain any inhibitory activity. In the lower panel, a Coomassie blue-stained SDS–15% polyacrylamide gel of the same frac- tions is shown using 5 μl of each fraction. The major protein of 25 kDa is indicated, and the minor bands of between 60 and 120 kDa are marked by arrowheads. (B) MonoQ-purified inhibitor preparation (2 μl) was separated on an SDS–15% polyacrylamide gel and stained with Coomassie blue. The migration of molecular size markers is shown. An adjacent lane containing the same amount of inhibitor was cut into individual fractions as indicated. An aliquot representing 5% of each fraction was added to a gel retardation assay using the HNF4 binding site, as given in Fig. 1.