FIG. 3.
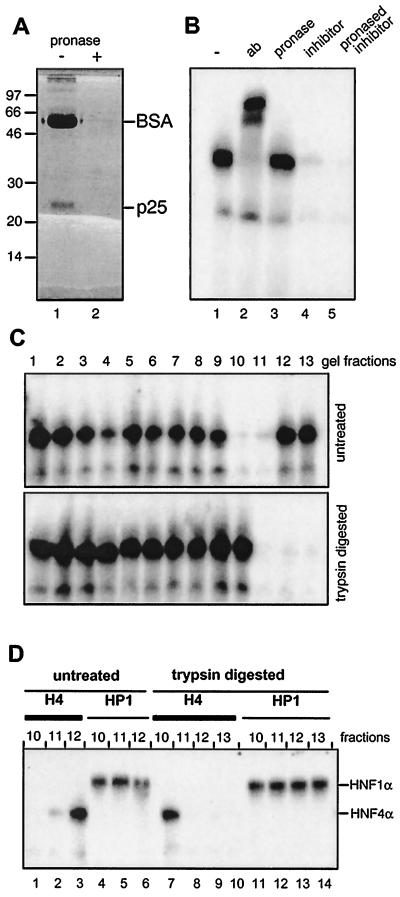
Protease digestion of the HNF4 inhibitor. (A) MonoQ-purified inhibitor preparation (2 μl) was combined with 20 μg of BSA and either separated directly on an SDS–15% polyacrylamide gel (lane 1) or digested with pronase prior to electrophoresis (lane 2). (B) Gel retardation assay using rat liver nuclear extract and the labeled HNF4 binding site as oligonucleotide is given in lane 1 and supplemented in lane 2 with the polyclonal antibody (ab) H4/66 specific for HNF4α (32). In lanes 3, 4, and 5, pronase-digested BSA, untreated HNF4 inhibitor, and pronase-digested inhibitor were added, respectively. (C) Untreated and trypsin-digested HNF4 inhibitor was separated in adjacent lanes of an SDS–15% polyacrylamide gel. The gel was cut into slices, and the HNF4 inhibitor was eluted and assayed in a gel retardation assay as given in Fig. 2B. (D) Aliquots of the indicated gel fractions of the untreated and trypsin-digested lanes of panel C were assayed in a gel retardation assay using the labeled oligonucleotide representing either the HNF4 (H4) or the HNF1 (HP1) binding site. The DNA-protein complexes containing HNF1α and HNF4α are indicated.