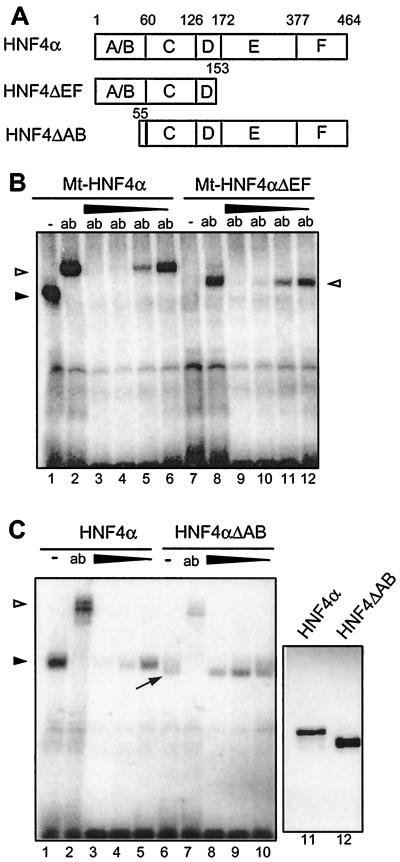
FIG. 5.
Effect of the HNF4 inhibitor on various HNF4 deletion constructs in gel retardation assays. (A) Schematic drawing of the deletion constructs used. Domains C and E represent the DNA-binding and the potential ligand-binding domains of HNF4α, respectively. (B) Nuclear extracts from HEK 293 cells transfected with the Myc-tagged full-length HNF4α (lanes 1 to 6) or with the C-terminal deletion construct Mt-HNF4αΔEF (lanes 7 to 12) were analyzed in gel retardation assays using the labeled HNF4 binding site. The addition of the monoclonal Myc tag-specific antibody 9E10 is shown (ab). A protein-free inhibitor preparation was added (1, 0.3, 0.1, or 0.03 μl) in lanes 3 to 6 and 9 to 12, respectively. The arrowhead marks the HNF4α-DNA complex, and the open triangles indicate the complex supershifted by the antibody. (C) Nuclear extracts from HEK 293 cells transfected with the full-length HNF4α (lanes 1 to 5) or with the N-terminal deletion construct HNF4αΔAB were analyzed in gel retardation assays using the labeled HNF4 binding site. The polyclonal antibody XH4α specific for HNF4α (13) was added in lanes 2 and 7 (ab). The protein-free inhibitor preparation was used at 1, 0.3, or 0.1 μl in lanes 3 to 5 and 8 to 10, respectively. The arrowhead marks the HNF4α-DNA complex, and the open triangle marks the complex supershifted by the antibody. The unusually broad protein-DNA complex containing HNF4αΔAB is marked by an arrow. Lanes 11 and 12 show the abundance of the transfected HNF4α proteins in 3 μl of HEK 293 cell extract in a Western blot of an SDS–12% polyacrylamide gel using monoclonal antibody H4/39f (32).