Fig. 5 |. Antitumour activity of drug candidates in mouse breast tumour models.
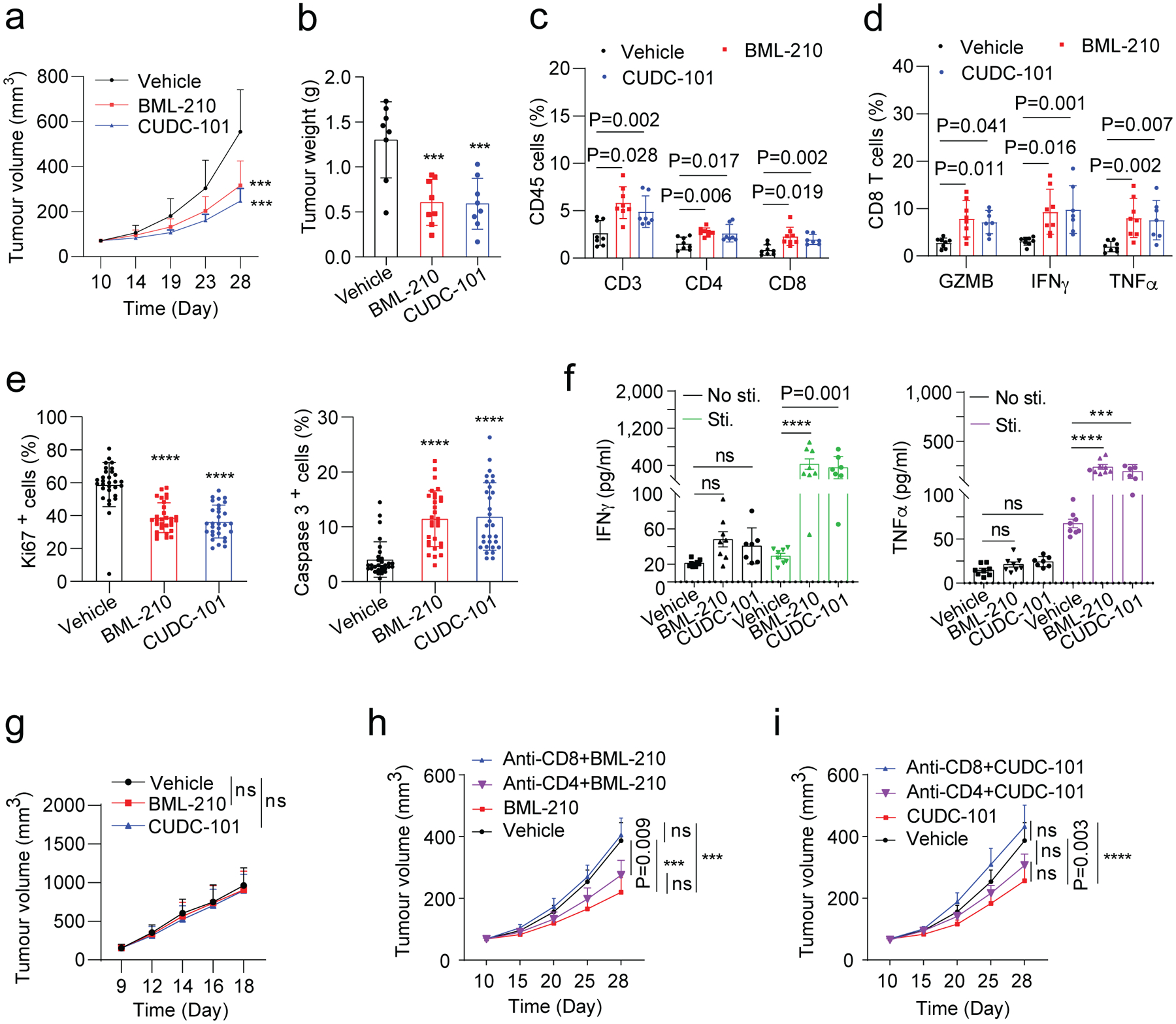
a,b, Gross dissected mammary tumour growth (a) and weight (b) of the EO771 tumours from the tumour-bearing C57BL/6 mice treated with vehicle control, BML-210 (20 mg kg−1), or CUDC-101 (20 mg kg−1). c, Proportions of total T, CD4+ T, and CD8+ T cells in total immune (CD45+) cells in the EO771 tumours from mice treated with control, BML-210 or CUDC-101. d, Percentage of active cells in total CD8+ T cells, indicated by GZMB+, IFNγ+, TNFα+ in flow cytometry analysis. e, Quantitative results for tumour cell proliferation (Ki67+) and apoptosis (Caspase 3+) in the tumours analyzed by IHC staining images (Extended Data Fig.3c). f, Levels of secreted IFNγ and TNF-α from CD8+ T cells isolated from the tumour tissues with and without PMA and ionomycin stimulation. g, Gross dissected mammary tumour growth of the EO771 tumours from the tumour-bearing immunodeficient nude mice treated with vehicle control, BML-210 (20 mg kg−1), or CUDC-101 (20 mg kg−1). Tumours from immunodeficient mice were harvested at day 18 post injection. h,i, Gross mammary tumour growth of the EO771 tumours from the tumour-bearing C57BL/6 mice treated with isotype control, CUDC-101 (20 mg kg−1), BML-210 (20 mg kg−1), anti-CD8 (10 mg kg−1) + CUDC-101 (20 mg kg−1), anti-CD8 (10 mg kg−1) + BML-210 (20 mg kg−1), anti-CD4 (10 mg kg−1) + CUDC-101 (20 mg kg−1) or anti-CD4 (10 mg kg−1) + BML-210 (20 mg kg−1). Tumours from tumour-bearing C57BL/6 mice were harvested at day 28 post injection. For statistical analysis of data, one-way ANOVA test was used in (a,b,e,g-i), and two-way ANOVA test was used in (c,d,f). Data are presented as mean ± SD. ***, p < 0.001; ****, p < 0.0001; ns, no significance.
