Figure 1.
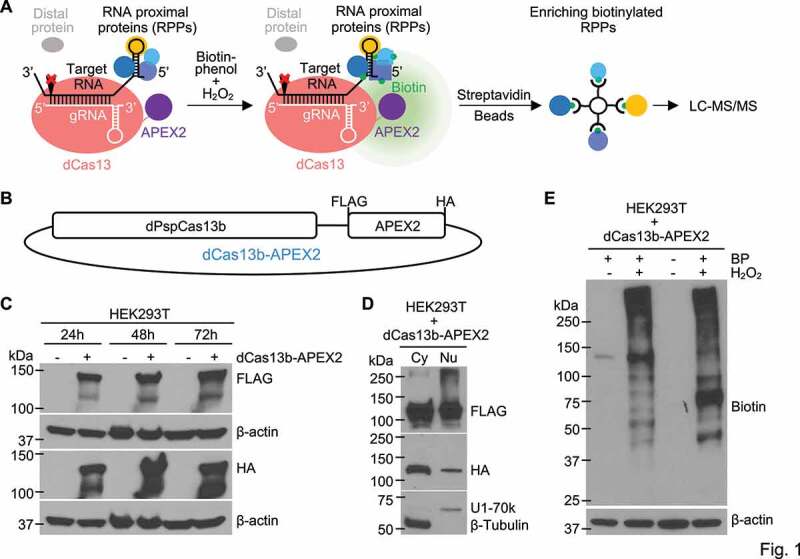
Designing and developing the RPL method. (a) Schematic illustration of the RPL workflow. A sequence-specific gRNA directs dCas13-APEX2 to target RNA and APEX2 in the fusion biotinylates target RNA proximal proteins in vivo in the presence of biotin-phenol and H2O2. Biotinylated RNA proximal proteins are then enriched using streptavidin beads and analysed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). (b) Diagram of the fusion protein dPspCas13b-FLAG-APEX2-HA (dCas13b-APEX2, or the RPL protein) expression construct. (c) Expression validation of the RPL protein by western blot. HEK293T cells transfected with or without the RPL plasmid were harvested 24 h-72 h post transfection and whole cell lysates were blotted with an anti-FLAG or anti-HA antibody. (d) The RPL protein is expressed in both the cytoplasm and the nucleus. HEK293T cells transfected with the RPL plasmid for 24 h were fractionated into cytoplasmic (Cy) and nuclear (Nu) fractions. Fractionation efficiency was evaluated by blotting for cytoplasmic protein β-Tubulin and nuclear protein U1-70k. (e) Validation of enzymatic activity of APEX2 in the RPL protein. HEK293T cells transfected with the RPL plasmid were treated with different combinations of biotin-phenol (BP) and H2O2. Proximity labelling was performed using different batches of HEK293T cells for lane 2 and lane 4. Whole cell lysates were blotted with anti-biotin antibody. β-actin in (C) and (E) was used as loading control
