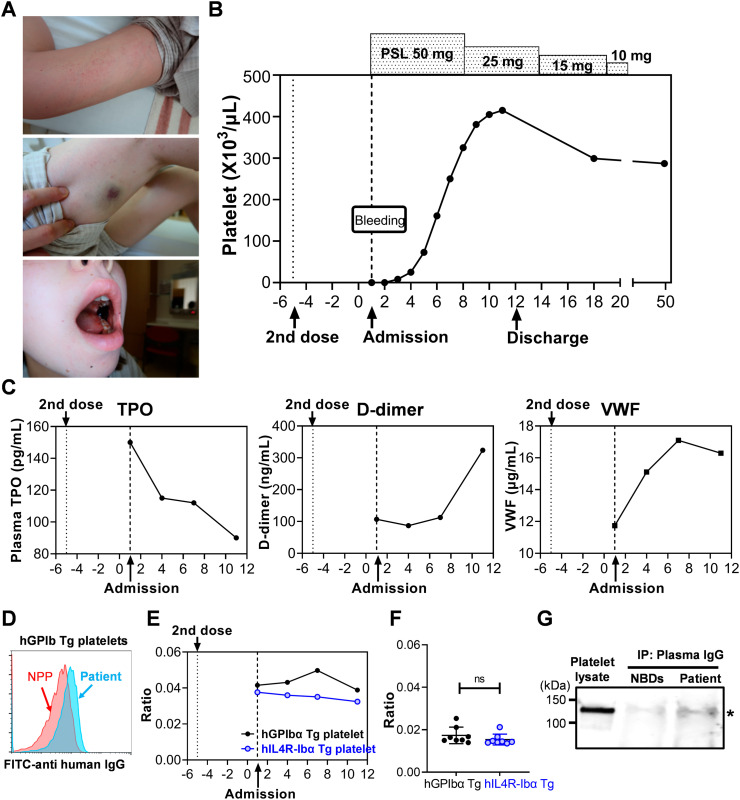
Fig. 1.
Clinical course and testing results of the patient. (A, B) Initial presentation of the bleeding symptoms on admission and platelet count responses to treatment. (C) Plasma levels of TPO, D-dimer, and VWF. TPO level in the plasma was measured using Human Thrombopoietin ELISA Kit (Sigma-Aldrich, St. Louis, MO) according to manufacturer's instruction. The minimal detectable dose of human TPO using this assay kit is 90 pg/mL. D-dimer was measured using monoclonal antibodies (DD189cc and biotinylated DD255cc; HyTest, Turku, Finland). Plasma D-dimer levels in the normal donors tested by this assay ranged from 26.7 to 142.7 ng/mL (mean 78.2 ± 39.2, n = 15). VWF was measured using anti-human VWF monoclonal antibody (2.2.9, produced at The Scripps Research Institute) and rabbit anti-human VWF polyclonal antibody (produced at The Scripps Research Institute). Plasma VWF levels in the normal donors tested by this assay ranged from 7.2 to 13.2 μg/mL (mean 10.4 ± 2.3, n = 8). (D) Flow cytometry analysis of the patient's plasma or normal pooled plasma (NPP; George King Bio-Medical) binding to hGPIbα Tg mouse platelets. Blood samples collected from transgenic mouse strains hGPIbα Tg and hIL4R-Ibα Tg were fixed with 1% paraformaldehyde and incubated with plasma samples of the patient or normal controls and then, bound IgG was detected with FITC-anti-human IgG (Biolegend, San Diego CA). Platelet population was gated by staining with APC-anti-mCD41 antibody (MWReg30, Biolegend). Samples were analyzed on a Novocyte flow cytometer (ACEA Biosciences), and the results were analyzed with FlowJo software. (E, F) Patient's plasma was incubated with hGPIbα Tg or hIL4R-Ibα Tg platelets, and the bound IgG was detected by FITC-anti-human IgG. Platelet population was gated by staining with APC-anti-mCD41 antibody. Median Fluorescence Intensity (MFI) of FITC-anti-human IgG was divided by MFI of APC-anti-mCD41 signal to correct platelet size difference, and the relative binding ratio are plotted. Results of the plasma samples obtained from normal healthy donors (n = 8) are shown in (F). (G) Immunoprecipitation and Western blot analysis of platelet lysate using Protein G agarose bearing IgG of the patient or normal healthy donors (NBDs). Plasma samples collected from the patient at four time points were pooled (total volume of 360 μL) and incubated with Protein G agarose beads. After washing, IgG bearing Protein G beads were washed and incubated with normal donor platelet lysates solubilized by 1% NP-40, then coprecipitated proteins were analyzed by sodium dodecyl sulfate gel electrophoresis (SDS-PAGE) followed by Western blotting. PVDF membrane was probed with anti- glycocalicin polyclonal antibody, and the signal was detected with IRDye 800 goat anti-rabbit IgG (LI-COR Biosciences, Lincoln, NE). The image was obtained using LI-COR Odyssey imaging system.