Abstract
Background
Although some studies have reported a decrease in preterm birth following the start of the COVID-19 pandemic, the findings are inconsistent.
Objective
This study aimed to compare the incidences of preterm birth before and after the introduction of COVID-19 mitigation measures in Scandinavian countries using robust population-based registry data.
Study Design
This was a registry-based difference-in-differences study using births from January 2014 through December 2020 in Norway, Sweden, and Denmark. The changes in the preterm birth (<37 weeks) rates before and after the introduction of COVID-19 mitigation measures (set to March 12, 2020) were compared with the changes in preterm birth before and after March 12 from 2014 to 2019. The differences per 1000 births were calculated for 2-, 4-, 8-, 12-, and 16-week intervals before and after March 12. The secondary analyses included medically indicated preterm birth, spontaneous preterm birth, and very preterm (<32 weeks) birth.
Results
A total of 1,519,521 births were included in this study. During the study period, 5.6% of the births were preterm in Norway and Sweden, and 5.7% were preterm in Denmark. There was a seasonal variation in the incidence of preterm birth, with the highest incidence during winter. In all the 3 countries, there was a slight overall decline in preterm births from 2014 to 2020. There was no consistent evidence of a change in the preterm birth rates following the introduction of COVID-19 mitigation measures, with difference-in-differences estimates ranging from 3.7 per 1000 births (95% confidence interval, −3.8 to 11.1) for the first 2 weeks after March 12, 2020, to −1.8 per 1000 births (95% confidence interval, −4.6 to 1.1) in the 16 weeks after March 12, 2020. Similarly, there was no evidence of an impact on medically indicated preterm birth, spontaneous preterm birth, or very preterm birth.
Conclusion
Using high-quality national data on births in 3 Scandinavian countries, each of which implemented different approaches to address the pandemic, there was no evidence of a decline in preterm births following the introduction of COVID-19 mitigation measures.
Key words: COVID-19, pregnancy outcomes, preterm birth, retrospective, Scandinavia
Introduction
A growing number of studies have attempted to assess the indirect consequences of the COVID-19 pandemic on key health indicators. It has been speculated that 1 of these indirect consequences is an impact on the birth outcomes, including a change in the prevalence of preterm birth. The suggested potential mechanisms for such an impact include hypotheses about improved air quality (because of strict lockdown measures), prevention of infections that may otherwise trigger preterm labour1, 2, 3, and changes to health-seeking behavior. In contrast, pregnant women have experienced added anxiety about COVID-19 infection alongside the negative impacts of unemployment and income insecurity, working from home, home-schooling, and reduced social support.4, 5, 6 In addition, many settings experienced changes in healthcare access and availability.7 A recent meta-analysis identified 16 studies assessing the impact of the COVID-19 pandemic on preterm birth, 12 of which were conducted in high-income countries (HIC).8 Although these individual studies reported conflicting findings, a subgroup analysis of the HIC studies suggested some evidence of a significant decrease in the incidence of preterm birth following the start of the COVID-19 pandemic. Most existing studies are based on data from selected healthcare facilities or are limited to regional data, and are therefore, small, potentially underpowered, and not representative of the general population. In addition, temporal and seasonal trends in preterm birth9 have not always been adequately accounted for. There continues to be insufficient evidence to conclude the impact of COVID-19 mitigation measures on preterm birth,10 particularly when focusing on longer periods of lockdown and specific preterm birth subtypes.
AJOG at a Glance.
Why was this study conducted?
This study aimed to assess the impact of COVID-19 mitigation measures on the incidence of preterm birth.
Key findings
In this difference-in-differences analysis of births in Scandinavia, there was no evidence of a change in the incidence of preterm birth following the initial introduction of COVID-19 mitigation measures in 2020.
What does this add to what is known?
Previous studies have reported conflicting findings. These studies have predominantly been based on data from healthcare facilities and are potentially underpowered and unrepresentative, and they have not always accounted for temporal trends in preterm birth.
This analysis of national registry data from 3 countries with varied levels of “lockdowns” provides no evidence of an indirect impact of the COVID-19 pandemic on preterm birth.
Norway, Sweden, and Denmark are similar countries in many ways, particularly in terms of universal healthcare, levels of income inequality, and fertility patterns. At the time when COVID-19 was first designated a pandemic by the World Health Organization (March 13, 2020), the COVID-19 rates were similarly low in all the 3 countries. Subsequently, each country pursued policy measures in an attempt to minimize the impact of COVID-19, with both Norway and Denmark introducing relatively strict lockdown measures in mid-March, whereas the approach in Sweden was initially somewhat less restrictive.11, 12, 13 All the 3 countries saw substantial changes in the behavior of citizens from mid-March onwards, with decreasing use of public transportation, less workplace commuting, and more time spent at home.14 The available behavioral indicators suggest that the strict lockdowns of Norway and Denmark translated into larger behavioral changes than those in Sweden.15
With national registry-based data from Norway, Sweden, and Denmark, we used a difference-in-differences (DiD) design to assess the impact of COVID-19 mitigation measures on the incidence of preterm birth.
Materials and Methods
Data sources and study population
Records of births at ≥22 weeks’ gestation occurring between January 1, 2014 and December 31, 2020 were obtained from the Medical Birth Registry of Norway,16 the Swedish Pregnancy Register,17 the Danish Medical Birth Register,18 the Danish National Patient Registry,19 and the Danish Civil Registration System.20 In Norway and Denmark, all births are included in the registry sources; in Sweden, 92% of the births are included in the national register. Further details of the data sources are listed in the appendix (Supplemental Table 1). Births with multiples were counted as one record only.
Ethical approval
This study was approved by the Regional Committee for Medical and Health Research Ethics of South/East Norway (approval number 141135) and the Swedish Ethical Review Authority (approval numbers: dnr 2020-01499, dnr 2020-02468, dnr 2021-00274). Each committee provided a waiver of consent for the participants. In Denmark, the study was registered with the Danish Data Protection Agency via the University of Southern Denmark (registration number 364 20/17416) and via Statistics Denmark.
Exposure
The DiD design requires a time point on which to split between an unexposed ‘pre’ period and an unexposed ‘post’ period. Although the intensity and timing of COVID-19 mitigation measures differed between the 3 countries, most of the measures were introduced around March 12, 2020 (Table 1 ). Thus, March 12, 2020 was used as the cutoff date for all the 3 countries.
Table 1.
Summary of early COVID-19 mitigation measures in Norway, Sweden, and Denmark
| Mitigation measures | Norway | Sweden | Denmark |
|---|---|---|---|
| Kindergarten or daycare and primary schools closed | March 12 | n/a | March 16 |
| High-school and universities closed | March 12 | March 17: recommendation | March 13 |
| Restrictions on gathering | March 12 | March 11 (500+) March 27 (50+) |
March 11 (100+) March 17 (10+) |
| Workplace closures | March 10: recommendation to work from home | March 16: recommendation to work from home | March 13: non-essential workers in the public sector ordered to stay home, private sector urged to allow home working |
| Non-essential businesses closed | Some closures from March 12 | — | Some closures from March 18, including restaurants/bars |
| Stay at home recommendations | March 12: avoid public transport and unnecessary travels, March 19: not allowed to spend night in vacation homes outside home county |
March 16: for over 70s March 19: avoid unnecessary travels |
March 11: restrict public transport and unnecessary travels |
| Restriction on internal movement | March 12 | March 19 | April 9 |
| Restrictions on international travel | March 13: recommendations to avoid all international travel, mandatory quarantine when arriving in Norway, isolation if symptoms | March 14: advice against all international travels, isolation and get tested if symptoms after arrival to Sweden | March 11: flights from high-risk areas cancelled March 14: all borders closed |
| Cancellation of public events | March 12 | March 12 | March 13 |
n/a, not applicable.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Preterm birth
We defined preterm birth as the birth of at least 1 live or stillborn infant before 37 completed weeks of pregnancy. Preterm birth was further stratified into medically indicated preterm birth (resulting from induction of labor or a prelabor cesarean delivery) or spontaneous preterm birth (birth after a spontaneous onset of labor). We included very preterm birth (<32 weeks) as an additional outcome. Further details on the definition of outcomes are included in the appendix (Supplemental Table 1).
Statistical analysis
The DiD design mimics experimental methods by comparing changes in an exposed group with those in an unexposed group.21 Specifically, we exploit the exogenous nature of the mid-March lockdown: everyone is exposed. However, because the exposure is fixed in time (mid-March 2020), the naïve comparison of before and after the introduction of lockdown measures might be confounded by any factor that is correlated with time, eg seasonal effects or changes in the characteristics of pregnant women. In the DiD design, this is solved by comparing the changes before and after March 12 not only in 2020 but also in the previous years. In this study, we compared the rate of preterm birth in the weeks before and after the introduction of COVID-19 mitigation measures in 2020 (March 12, difference 1) with the difference in the preterm birth rates before and after March 12 in earlier years (2014–2019, difference 2). The DiD estimate is the difference between these 2 differences, obtained using linear probability models with robust standard errors and presented as a risk difference in points per 1000 births. Statistically, we use an interaction term between pre-post lockdown and year to derive the DiD estimate. By including the year and week fixed effects, this approach accounts for the background trends in the birth outcomes,22 including seasonal trends. The DiD estimate can be interpreted as the change in birth outcomes that are related to the implementation of COVID-19 mitigation measures in the various countries, beyond the background trends in season and year. If there is no relationship between the COVID-19 mitigation measures and the subsequent birth outcomes, then the DiD estimate would be equal to 0. We accounted for clustering by mother where this information was available (Norway and Sweden). To allow for a time lag between the introduction of the COVID-19 mitigation measures and a potential impact on preterm birth, we modeled 5 different time intervals as follows: 2 weeks after March 12 compared with 2 weeks before and similar comparisons for intervals of 4, 8, 12, and 16 weeks. We first ran a model for any preterm birth, and then we ran additional models for medically indicated preterm birth, spontaneous preterm birth, and very preterm birth. The parallel trends assumption was explored using visual inspection of pre-trends.
Individual data sharing was not possible between countries because of privacy restrictions; therefore, the DiD analyses were conducted within each country separately according to a standardized common study protocol. The pooled DiD estimates were generated using a random-effects meta-analysis with inverse variance weighting of individual-country results. Heterogeneity was assessed using the I 2 statistic, calculated as 100%×(Q–df)/Q, where Q is Cochrane's heterogeneity statistic and df denotes degrees of freedom.23 The analyses were performed using SAS EG version 9.4 (SAS Institute, Cary, NC) and Stata version 16 (StataCorp, College Station, TX).
Results
There were 1,552,401 births between 2014 and 2020 in the 3 countries. After excluding 32,880 births with missing gestational lengths, gestational age <22 weeks, unknown outcome, or second or higher order births from a multiple pregnancy, 1,519,521 births were included in our study population (392,586 in Norway, 713,121 in Sweden, and 413,814 in Denmark; Supplemental Figure 1). The proportion of preterm birth (<37 completed weeks) was similar across all the 3 countries: 5.6% in Norway, 5.6% in Sweden, and 5.7% in Denmark (Table 2 ). In all the 3 countries, there was a slight decline in the proportion of preterm birth between 2014 and 2020 (Supplemental Table 2, Supplemental Table 3, Supplemental Table 4).
Supplemental Figure 1.
Study flowchart
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Table 2.
Characteristics of included births from 2014 to 2020 in Norway, Sweden, and Denmark
| Characteristics | Norway |
Sweden |
Denmark |
|||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| All births | 392,586 | 713,121 | 413,814 | |||
| Gestational age (wk) | ||||||
| Extremely preterm <28 | 1449 | (0.4) | 2670 | (0.4) | 1620 | (0.4) |
| Very preterm 28–<32 | 2123 | (0.5) | 3912 | (0.5) | 2393 | (0.6) |
| Moderate/late preterm 32–<37 | 18,256 | (4.7) | 33,264 | (4.7) | 19,411 | (4.7) |
| Term 37–<42 | 354,821 | (90.4) | 636,182 | (89.2) | 381,218 | (92.1) |
| Postterm ≥42 | 15,937 | (4.1) | 36,113 | (5.1) | 9172 | (2.2) |
| Maternal age | ||||||
| <20 | 3710 | (0.9) | 7266 | (1.0) | 3296 | (0.8) |
| 20–24 | 41,279 | (10.5) | 75,668 | (10.6) | 41,652 | (10.1) |
| 25–29 | 126,280 | (32.2) | 223,444 | (31.3) | 138,920 | (33.6) |
| 30–34 | 139,841 | (35.6) | 246,949 | (34.6) | 144,304 | (34.9) |
| 35–39 | 66,785 | (17.0) | 128,099 | (18.0) | 69,390 | (16.8) |
| ≥40 | 14,690 | (3.7) | 31,484 | (4.4) | 16,252 | (3.9) |
| Missing | 1 | (0.0) | 211 | (0.0) | ||
| Parity | ||||||
| 0 | 166,742 | (42.5) | 306,085 | (42.9) | 190,650 | (46.1) |
| ≥1 | 225,844 | (57.5) | 402,892 | (56.5) | 223,120 | (53.9) |
| Missing | 4144 | (0.6) | 44 | (0.0) | ||
| Multiple birth | ||||||
| Yes | 6107 | (1.6) | 10,072 | (1.4) | 6768 | (1.6) |
| No | 386,479 | (98.4) | 703,049 | (98.6) | 407,046 | (98.4) |
| Season of conceptiona | ||||||
| Winter | 90,360 | (23.0) | 186,013 | (26.1) | 105,919 | (25.6) |
| Spring | 92,381 | (23.5) | 189,348 | (26.6) | 97,751 | (23.6) |
| Summer | 102,690 | (26.2) | 170,177 | (23.9) | 100,506 | (24.3) |
| Fall | 107,155 | (27.3) | 167,583 | (23.5) | 109,638 | (26.5) |
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Winter (December–February); Spring (March–May); Summer (June–August); Fall (September–November).
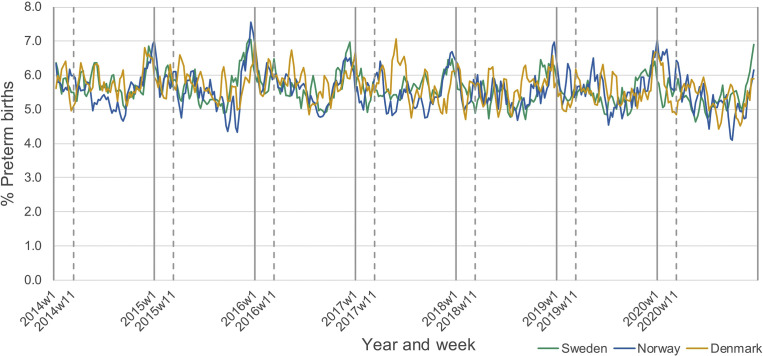
Figure 1 presents the weekly incidence (using a 3-week rolling average) of preterm birth between January 2014 and December 2020, with week 11 (which includes the cutoff date, March 12) indicated by a vertical dashed line. There was a clear general seasonal trend in preterm birth, with the incidence peaking in the early winter months, and the lowest levels observed in late summer and early fall. Notably, in most years, the incidence of preterm birth steadily declined during the first 3 months of each year.
Figure 1.
Incidence of preterm birth by weeka from 2014 to 2020 in Norway, Sweden, and Denmark
aRolling 3-week average. Dashed vertical lines represent week including March 12.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
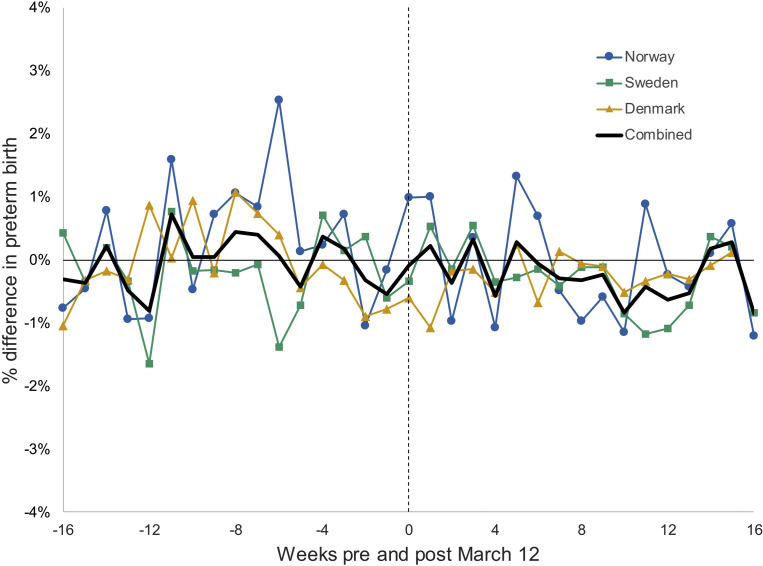
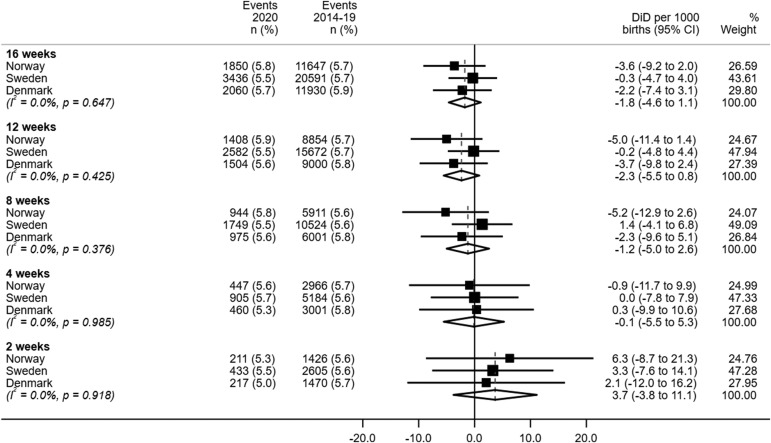
The DiD analyses included 895,945 births occurring in the period 16 weeks before and after March 12 from 2014 to 2020 (234,517 in Norway, 421,544 in Sweden, and 239,884 in Denmark). There was no evidence that the parallel trends assumption was violated in any of the 3 countries (Figure 2 ; Supplemental Figure 2). The DiD estimates for preterm birth with different weekly intervals are presented in Figure 3 (source data in Supplemental Table 5, Supplemental Table 6, Supplemental Table 7). For all time intervals, there was no discernible difference in the country-specific incidence of preterm birth after lockdown. There was no evidence of heterogeneity in the meta-analysis, and pooled estimates did not show an overall decrease across the 3 countries.
Figure 2.
Percent difference in preterm birth in the weeks before and after March 12a, comparing births in 2020 to births in 2014 to 2019 in Norway, Sweden, and Denmark
aWeek beginning March 12 represented by a dashed vertical line.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
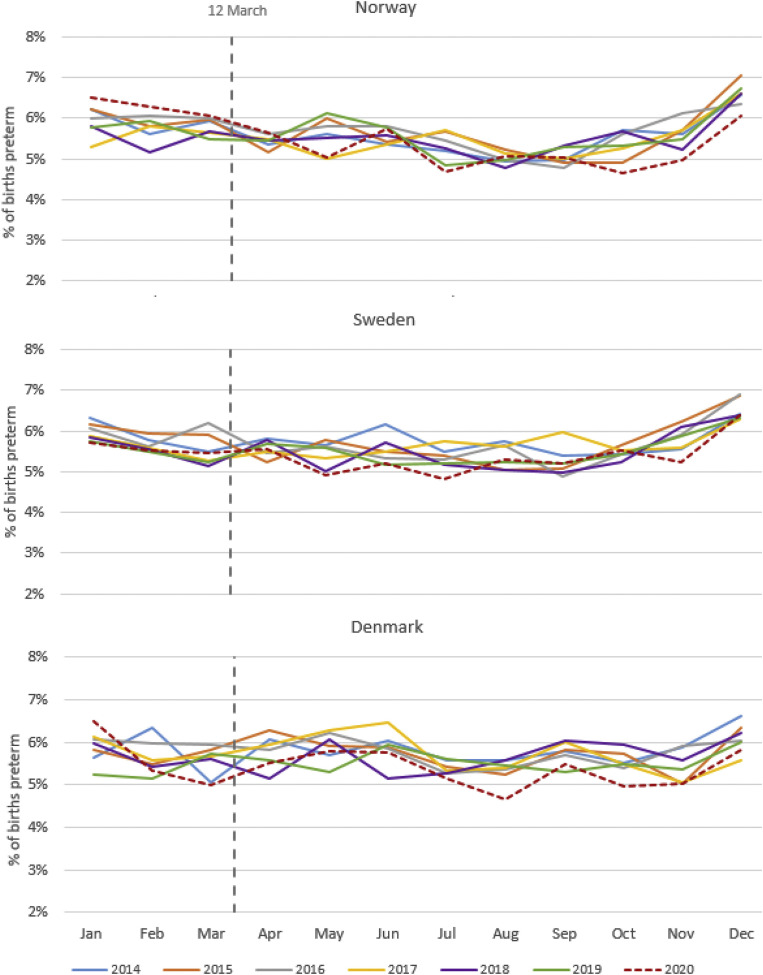
Supplemental Figure 2.
Preterm birth by month and year in Norway, Sweden, and Denmark, 2014–2020
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Figure 3.
Meta-analyses of difference-in-differences estimates for preterm birth
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
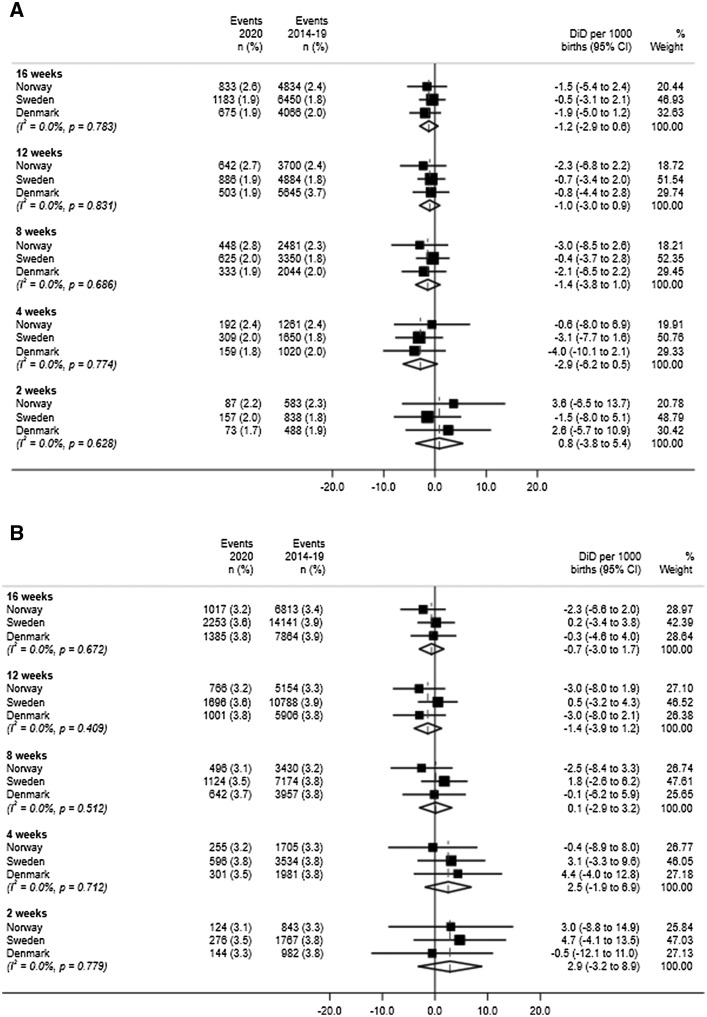
Similarly, when preterm birth was stratified into medically indicated or spontaneous, there was no convincing difference in the country-specific prevalence following March 12, 2020 in any of the 3 countries (Figure 4 ). As with the overall preterm birth analysis, there was no evidence of heterogeneity, and pooled estimates did not provide evidence of a change in the incidence of either medically indicated or spontaneous preterm birth.
Figure 4.
Meta-analyses of difference-in-differences estimates
For (A) medically indicated preterm birth and (B) spontaneous preterm birth.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
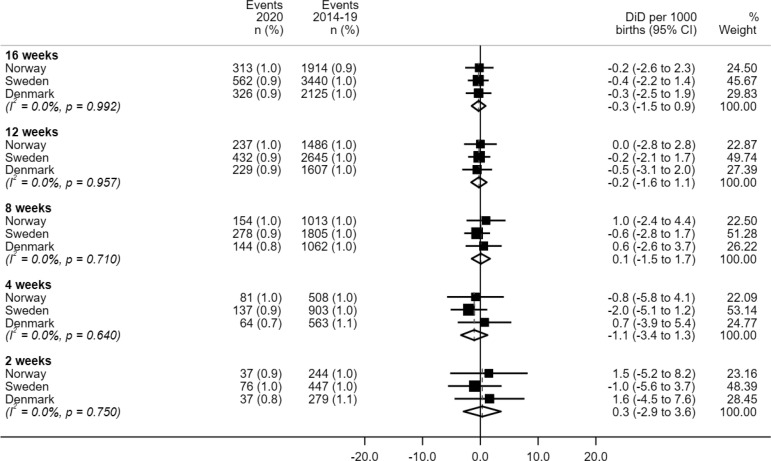
The introduction of COVID-19 mitigation measures had no impact on the incidence of very preterm birth (<32 completed weeks) in any of the 3 countries (Supplemental Figure 3).
Supplemental Figure 3.
Meta-analyses of difference-in-differences estimates for very preterm birth
CI, confidence interval; DiD, difference-in-differences.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Comment
Principal findings
We found no convincing evidence to support a change in the incidence of preterm birth following the introduction of COVID-19 mitigation measures in Norway, Sweden, and Denmark. Similarly, the rates of very preterm birth (<32 completed weeks) did not seem to decline after lockdown in any of the Scandinavian countries. The findings were similar when evaluating medically indicated or spontaneous preterm births separately.
Results in the context of what is known
There have been reports of a decline in preterm births after the onset of the COVID-19 pandemic in HICs8 , 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, although findings are inconsistent.37, 38, 39, 40, 41, 42 Pooled estimates from a recent meta-analysis suggest a modest decrease in overall preterm birth in HICs only and also a reduction in spontaneous preterm birth but not medically indicated preterm birth,8 although the latter finding rests on the results from only 2 hospital-based studies.25 , 37 Notably, an earlier analysis of Danish data comparing births in the month following lockdown to births in the same interval in earlier years concluded that there was a decrease in extremely preterm birth after lockdown but no similar trend for later preterm births.27 However, this was on the basis of only 1 extremely preterm birth recorded for the 2020 study period. A short report comparing births in Sweden before and after the start of the COVID-19 pandemic did not find any association between birth during the COVID-19 pandemic and preterm birth,42 which is consistent with the findings reported here. The general inconsistency in results across previous studies likely reflects methodological heterogeneity, selection criteria, and a lack of ability to minimize bias caused by existing seasonal and time trends in preterm birth, and also low power for rare outcomes such as preterm birth subtypes.10 In addition, inconsistencies in the results may reflect heterogeneity in the mitigation measures and differing population and health system characteristics.
Although the 3 Scandinavian countries have a similar culture, populations, and healthcare systems, at the beginning of the pandemic, there was a major difference in the approach to policies and interventions designed to mitigate the COVID-19 pandemic.12 , 13 Both the Norwegian and Danish governments swiftly introduced emergency legislative powers, allowing them to implement domestic restrictions that would otherwise be constitutionally unlawful. One key difference between the 3 countries relates to education closures: in mid-March 2020, all schools were closed in Norway and Denmark, whereas Sweden followed some days later with only a recommendation for high schools and universities to close. There was also stronger advice to work from home in both Norway and Denmark. Although the 3 countries had similar rates of COVID-19 cases on March 12, by July 2—16 weeks into the pandemic—the cumulative confirmed COVID-19 deaths per million people was 46.3 in Norway, 104.62 in Denmark, and 535.8 in Sweden.14 Trust in the government is generally high across all the 3 countries,43 and there is evidence of high compliance with the mitigation measures that were introduced as a result of the pandemic.44 Adherence to public health recommendations around social distancing and hygiene almost certainly contributed to an abrupt end to the 2019/20 influenza season in the 3 countries,45 with some evidence that these measures also contributed to a decrease in non-COVID-19 respiratory infections.46 Although there were likely some changes to healthcare in the 3 countries immediately following the start of the pandemic, these were likely to predominantly be reflected in reductions in elective care rather than changes in the provision of essential maternal health services.
Although the results from the meta-analyses lacked evidence for a decrease in preterm birth for any of the defined time intervals, it is notable that in Norway, the estimates were negative (suggesting a decrease after March 12, 2020) for the overall preterm birth outcome for the 8-, 12-, and 16-week intervals. The fact that these trends were only observed for the longer time intervals following March 12, 2020 in Norway may support the hypothesis of a gradual change in biologic processes that influence preterm birth rather than any immediate impact of changes in healthcare delivery. However, the fact that the trends for Denmark—which arguably had a similar level of “lockdown”—were much weaker does not support this hypothesis of some gradual change in the incidence of preterm birth after the introduction of stricter COVID-19 mitigation measures.
Clinical and research implications
Although there are some well-known risk factors for preterm birth, the biologic mechanisms behind preterm birth remain poorly understood,47 and identifying additional factors that could influence preterm risk is of great interest, as preterm births represent a substantial burden for the children themselves, the parents, and society. Early reports of a decrease in preterm birth following the onset of the COVID-19 pandemic have therefore ignited much interest,10 and this is likely in part because of the well-established challenge of further reducing preterm birth incidence in countries with already low rates of preterm birth.48 Further research could usefully investigate the extent to which the impact of COVID-19 mitigation measures may be mediated by contextual factors such as existing trends in preterm birth and characteristics of healthcare systems.
Strengths and limitations
This study used national registry data covering more than 1.5 million births in the 3 Scandinavian countries from 2014 through 2020. We captured all births in Norway and Denmark in this time period, and 92% of births in Sweden. Approximately 8% of births were missing because of incomplete electronic data transfer in 3 of Sweden’s 21 counties.17 The missing registrations did not depend on the birth outcomes and would not bias associations. By comparing the births around March 2020 with those in the same seasonal period in the previous years, we could account for discernible seasonal and yearly trends in preterm birth. Prospective and well-established routine collection of data reduces bias from reporting, and our primary outcome (preterm birth) is an objective outcome based on gestational age estimates derived predominantly from ultrasonography.
The COVID-19 pandemic arguably represents the most important natural experiments of our time and is well suited to the application of quasi-experimental methods. DiD methods are designed to minimize the effect of any unmeasured confounding. Nevertheless, unbiased DiD estimates hinge on the assumption of parallel pretrends. Visual inspection of plots did not suggest that the parallel trends assumption was violated. The validity of the approach also depends on the “common shocks” assumption, which can be defined as the assumption that any other event that occurs during or following the intervention should affect each group equally. The common shocks assumption is essentially an untestable assumption involving any exogenous shocks that may be unknown. However, the use of data from the 3 countries with comparable findings suggest that this is not the cause of our findings.
A strength of our study was that we could subdivide preterm births into those with a spontaneous onset and those that were medically indicated. We could also assess very preterm birth (<32 weeks) as a standalone outcome. However, the number of country-specific events by week was insufficient to assess any impact on less common preterm birth subtypes such as extremely preterm birth (<28 completed weeks). We could not therefore use our DiD approach to confirm the suggested decreased incidence of extremely preterm birth found in a previous Danish study.27
This study aimed to assess the indirect consequences of the COVID-19 pandemic on preterm birth, and we, therefore, did not include information on SARS-CoV-2 infection in pregnancy. There is emerging evidence that SARS-CoV-2 infection is associated with an increased risk of preterm birth.49 , 50 However, given the generally low level of testing among asymptomatic and mild cases, these findings predominantly relate to more severe infections, so it is expected that confounding by indication will bias the estimates toward an association. The impact of any direct effect of SARS-CoV-2 infection on preterm birth in Scandinavia is likely to be minimal, given the still comparatively low rates of infection in these countries during the study period.
Conclusion
The indirect impacts of the COVID-19 pandemic are far-reaching and are still only beginning to be understood. Using robust population-based data from 3 HIC with varying levels of COVID-19 mitigation measures, we found no strong evidence of a decline in preterm birth following the onset of the COVID-19 pandemic in March 2020.
Footnotes
The authors report no conflict of interest.
This research was supported by NordForsk (project number 105545) and the Research Council of Norway through its Centres of Excellence funding scheme (project number 262700). L.H.M is supported in part by grants from the Novo Nordisk Foundation (NNF17OC0027594, NNF17OC0027812). T.G.P is supported via funding awarded by the Danish Ministry of Higher Education and Science.
The funders had no role in the study design; the collection, analysis, and interpretation of data; writing of the report; or in the decision to submit the article for publication.
Cite this article as: Oakley LL, Örtqvist AK, Kinge J, et al. Preterm birth after the introduction of COVID-19 mitigation measures in Norway, Sweden, and Denmark: a registry-based difference-in-differences study. Am J Obstet Gynecol 2022;226:550.e1-22.
Appendix
Supplemental Table 1.
Outcome definitions and data sources
| Data sources or outcome | Norway | Sweden | Denmark |
|---|---|---|---|
| Data sources | Medical Birth Registry of Norway | Swedish Pregnancy Register | Danish National Patient Register The Danish Civil Registration System Danish Medical Birth Register |
| Preterm birth | Live birth or stillbirth <259 da | 1) ICD-10 O60.1 (Spontaneous preterm labor with preterm delivery) or 2) live birth or stillbirth <259 da | Live birth or stillbirth <259 da |
| Medically-indicated preterm birth | Live birth or stillbirth <259 d with induced labor or cesarean delivery without labor | 1) ICD-10 O60.3 (Preterm birth without spontaneous start of labor) or 2) live birth or stillbirth <259 d with induced labor or cesarean delivery without labor | Live birth or stillbirth <259 d with induced labor, or cesarean delivery without labor. If there is a code indicating rupture of membranes without regular contractions, the birth is reclassified as spontaneous preterm. |
| Spontaneous preterm birth | Live birth or stillbirth <259 d with spontaneous start of labor | 1) ICD-10 O60.1 (Spontaneous preterm labor with preterm delivery) or 2) live birth or stillbirth <259 d with spontaneous start | Live birth or stillbirth <259 d that is not classified as induced (provided above) and is not unclassifiable |
| Very preterm birth | Live birth or stillbirth <223 d | Live birth or stillbirth <223 d | Live birth or stillbirth <223 d |
ICD-10, International Classification of Diseases, Tenth Revision.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Gestational age is based on routine ultrasound measurements when this is available (approximately 98% of births); otherwise, the last menstrual period is used.
Supplemental Table 2.
Characteristics of births in Norway by year, 2014 to 2020
| Characteristics | All |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| All births | 392,586 | 58,548 | 58,417 | 58,563 | 56,123 | 54,734 | 54,053 | 52,148 | ||||||||
| Gestational age (wk) | ||||||||||||||||
| Extremely preterm <28 | 1449 | (0.4) | 250 | (0.4) | 232 | (0.4) | 209 | (0.4) | 203 | (0.4) | 195 | (0.4) | 194 | (0.4) | 166 | (0.3) |
| Very preterm 28–<32 |
2123 | (0.5) | 324 | (0.6) | 316 | (0.5) | 339 | (0.6) | 281 | (0.5) | 268 | (0.5) | 311 | (0.6) | 284 | (0.5) |
| Moderate or late preterm 32–<37 | 18,256 | (4.7) | 2685 | (4.6) | 2750 | (4.7) | 2789 | (4.8) | 2587 | (4.6) | 2539 | (4.6) | 2506 | (4.6) | 2400 | (4.6) |
| Term 37–<42 | 354,821 | (90.4) | 53,200 | (90.9) | 52,848 | (90.5) | 52,873 | (90.3) | 50,448 | (89.9) | 49,397 | (90.2) | 48,665 | (90.0) | 47,390 | (90.9) |
| Postterm ≥42 | 15,937 | (4.1) | 2089 | (3.6) | 2271 | (3.9) | 2353 | (4.0) | 2604 | (4.6) | 2335 | (4.3) | 2377 | (4.4) | 1908 | (3.7) |
| Maternal age | ||||||||||||||||
| <20 | 3710 | (0.9) | 808 | (1.4) | 741 | (1.3) | 659 | (1.1) | 489 | (0.9) | 414 | (0.8) | 349 | (0.6) | 250 | (0.5) |
| 20–24 | 41,279 | (10.5) | 7474 | (12.8) | 7097 | (12.1) | 6618 | (11.3) | 5790 | (10.3) | 5395 | (9.9) | 4755 | (8.8) | 4150 | (8.0) |
| 25–29 | 126,280 | (32.2) | 18,765 | (32.1) | 19,179 | (32.8) | 19,224 | (32.8) | 18,553 | (33.1) | 17,641 | (32.2) | 16,896 | (31.3) | 16,022 | (30.7) |
| 30–34 | 139,841 | (35.6) | 19,852 | (33.9) | 19,593 | (33.5) | 20,243 | (34.6) | 19,820 | (35.3) | 19,999 | (36.5) | 20,254 | (37.5) | 20,080 | (38.5) |
| 35–39 | 66,785 | (17.0) | 9558 | (16.3) | 9733 | (16.7) | 9666 | (16.5) | 9356 | (16.7) | 9295 | (17.0) | 9623 | (17.8) | 9554 | (18.3) |
| ≥40 | 14,690 | (3.7) | 2091 | (3.6) | 2074 | (3.6) | 2152 | (3.7) | 2115 | (3.8) | 1990 | (3.6) | 2176 | (4.0) | 2092 | (4.0) |
| Missing | 1 | 1 | ||||||||||||||
| Parity | ||||||||||||||||
| 0 | 166,742 | (42.5) | 24,754 | (42.3) | 24,920 | (42.7) | 24,901 | (42.5) | 23,624 | (42.1) | 23,168 | (42.3) | 22,999 | (42.5) | 22,376 | (42.9) |
| ≥1 | 225,844 | (57.5) | 33,794 | (57.7) | 33,497 | (57.3) | 33,662 | (57.5) | 32,499 | (57.9) | 31,566 | (57.7) | 31,054 | (57.5) | 29,772 | (57.1) |
| Missing | ||||||||||||||||
| Multiple birth | ||||||||||||||||
| Yes | 6107 | (1.6) | 937 | (1.6) | 982 | (1.7) | 935 | (1.6) | 898 | (1.6) | 821 | (1.5) | 826 | (1.5) | 708 | (1.4) |
| No | 386,479 | (98.4) | 57,611 | (98.4) | 57,435 | (98.3) | 57,628 | (98.4) | 55,225 | (98.4) | 52,913 | (96.7) | 53,227 | (98.5) | 51,440 | (98.6) |
| Country of birth | ||||||||||||||||
| Norway | 275,365 | (70.1) | 41,835 | (71.5) | 41,230 | (70.6) | 41,124 | (70.2) | 38,758 | (69.1) | 37,862 | (69.2) | 37,688 | (69.7) | 36,868 | (70.7) |
| Other Scandinavia | 8228 | (2.1) | 1213 | (2.1) | 1210 | (2.1) | 1243 | (2.1) | 1154 | (2.1) | 1155 | (2.1) | 1179 | (2.2) | 1074 | (2.1) |
| Outside Scandinavia | 107,318 | (27.3) | 15,187 | (25.9) | 15,703 | (26.9) | 15,957 | (27.2) | 15,953 | (28.4) | 15,526 | (28.4) | 14,982 | (27.7) | 14,010 | (26.9) |
| Missing | 1675 | (0.4) | 313 | (0.5) | 274 | (0.5) | 239 | (0.4) | 258 | (0.5) | 191 | (0.3) | 204 | (0.4) | 196 | (0.4) |
| Maternal education status (y) | ||||||||||||||||
| ≤9 | 58,273 | (14.8) | 8910 | (15.2) | 9037 | (15.5) | 8911 | (15.2) | 8477 | (15.1) | 8175 | (14.9) | 7773 | (14.4) | 6990 | (13.4) |
| 10–12 | 85,421 | (21.8) | 13,806 | (23.6) | 13,507 | (23.1) | 13,095 | (22.4) | 12,109 | (21.6) | 11,404 | (20.8) | 11,118 | (20.6) | 10,382 | (19.9) |
| >12 | 218,689 | (55.7) | 32,129 | (54.9) | 32,045 | (54.9) | 32,287 | (55.1) | 31,039 | (55.3) | 30,441 | (55.6) | 30,620 | (56.6) | 30,128 | (57.8) |
| Missing | 30,203 | (7.7) | 3703 | (6.3) | 3828 | (6.6) | 4270 | (7.3) | 4498 | (8.0) | 4714 | (8.6) | 4542 | (8.4) | 4648 | (8.9) |
| Season of conceptiona | ||||||||||||||||
| Winter | 90,360 | (23.0) | 13,352 | (22.8) | 13,492 | (23.1) | 13,304 | (22.7) | 12,928 | (23.0) | 12,648 | (23.1) | 12,580 | (23.3) | 12,056 | (23.1) |
| Spring | 92,381 | (23.5) | 13,979 | (23.9) | 13,628 | (23.3) | 13,772 | (23.5) | 13,198 | (23.5) | 12,837 | (23.5) | 12,789 | (23.7) | 12,178 | (23.4) |
| Summer | 102,690 | (26.2) | 15,405 | (26.3) | 15,081 | (25.8) | 15,641 | (26.7) | 14,734 | (26.3) | 14,271 | (26.1) | 13,946 | (25.8) | 13,612 | (26.1) |
| Fall | 107,155 | (27.3) | 15,812 | (27.0) | 16,216 | (27.8) | 15,846 | (27.1) | 15,263 | (27.2) | 14,978 | (27.4) | 14,738 | (27.3) | 14,302 | (27.4) |
| BMI (kg/m2) | ||||||||||||||||
| <18.5 | 12,941 | (3.3) | 1777 | (3.0) | 1858 | (3.2) | 1973 | (3.4) | 1984 | (3.5) | 1884 | (3.4) | 1821 | (3.4) | 1644 | (3.2) |
| 18.5–<25 | 200,623 | (51.1) | 26,170 | (44.7) | 27,091 | (46.4) | 29,939 | (51.1) | 29,713 | (52.9) | 29,851 | (54.5) | 29,524 | (54.6) | 28,335 | (54.3) |
| 25–<30 | 73,321 | (18.7) | 9008 | (15.4) | 9385 | (16.1) | 10,553 | (18.0) | 10,653 | (19.0) | 11,121 | (20.3) | 11,306 | (20.9) | 11,295 | (21.7) |
| ≥30 | 41,315 | (10.5) | 5047 | (8.6) | 5060 | (8.7) | 5665 | (9.7) | 5901 | (10.5) | 6230 | (11.4) | 6657 | (12.3) | 6755 | (13.0) |
| Missing | 64,386 | (16.4) | 16,546 | (28.3) | 15,023 | (25.7) | 10,433 | (17.8) | 7872 | (14.0) | 5648 | (10.3) | 5746 | (10.6) | 4119 | (7.9) |
| Smoking in early pregnancy | ||||||||||||||||
| No | 342,517 | (87.2) | 48,487 | (82.8) | 50,595 | (86.6) | 51,729 | (88.3) | 49,308 | (87.9) | 48,488 | (88.6) | 48,104 | (89.0) | 46,006 | (88.2) |
| Yes | 15,199 | (3.9) | 3678 | (6.3) | 2941 | (5.0) | 2530 | (4.3) | 1993 | (3.6) | 1665 | (3.0) | 1301 | (2.4) | 1091 | (2.1) |
| Missing | 34,870 | (8.9) | 6383 | (10.9) | 4881 | (8.4) | 4304 | (7.3) | 4822 | (8.6) | 4781 | (8.7) | 4648 | (8.6) | 5051 | (9.7) |
BMI, body mass index.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Winter (December–February); Spring (March–May); Summer (June–August); Fall (September–November).
Supplemental Table 3.
Characteristics of births in Sweden by year, 2014 to 2020
| Characteristics | All |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| All births | 713,121 | 89,437 | 102,483 | 107,877 | 104,314 | 104,556 | 102,937 | 101,517 | ||||||||
| Gestational age (wk) | ||||||||||||||||
| Extremely preterm <28 | 2670 | (0.4) | 374 | (0.4) | 401 | (0.4) | 432 | (0.4) | 370 | (0.4) | 349 | (0.3) | 385 | (0.4) | 359 | (0.4) |
| Very preterm 28–<32 |
3912 | (0.5) | 537 | (0.6) | 579 | (0.6) | 601 | (0.6) | 590 | (0.6) | 553 | (0.5) | 530 | (0.5) | 522 | (0.5) |
| Moderate or late preterm 32–<37 | 33,264 | (4.7) | 4231 | (4.7) | 4869 | (4.8) | 5095 | (4.7) | 4924 | (4.7) | 4822 | (4.6) | 4740 | (4.6) | 4583 | (4.5) |
| Term 37–<42 |
636,182 | (89.2) | 79,221 | (88.6) | 90,605 | (88.4) | 95,382 | (88.4) | 92,699 | (88.9) | 93,240 | (89.2) | 92,464 | (89.8) | 92,571 | (91.2) |
| Postterm ≥42 |
36,113 | (5.1) | 5074 | (5.7) | 6029 | (5.9) | 6367 | (5.9) | 5731 | (5.5) | 5592 | (5.3) | 4818 | (4.7) | 2482 | (2.4) |
| Maternal age | ||||||||||||||||
| <20 | 7266 | (1.0) | 1078 | (1.2) | 1139 | (1.1) | 1332 | (1.2) | 1073 | (1.0) | 1052 | (1.0) | 880 | (0.9) | 712 | (0.7) |
| 20–24 | 75,668 | (10.6) | 10,980 | (12.3) | 12,347 | (12.0) | 12,560 | (11.6) | 11,245 | (10.8) | 10,551 | (10.1) | 9542 | (9.3) | 8443 | (8.3) |
| 25–29 | 223,444 | (31.3) | 27,130 | (30.3) | 32,283 | (31.5) | 34,390 | (31.9) | 33,200 | (31.8) | 33,358 | (31.9) | 32,116 | (31.2) | 30,967 | (30.5) |
| 30–34 | 246,949 | (34.6) | 30,619 | (34.2) | 34,198 | (33.4) | 35,722 | (33.1) | 35,387 | (33.9) | 36,401 | (34.8) | 36,762 | (35.7) | 37,860 | (37.3) |
| 35–39 | 128,099 | (18.0) | 15,742 | (17.6) | 18,126 | (17.7) | 19,087 | (17.7) | 18,713 | (17.9) | 18,719 | (17.9) | 18,864 | (18.3) | 18,848 | (18.6) |
| ≥40 | 31,484 | (4.4) | 3846 | (4.3) | 4350 | (4.2) | 4739 | (4.4) | 4667 | (4.5) | 4459 | (4.3) | 4750 | (4.6) | 4673 | (4.6) |
| Missing | 211 | (0.0) | 42 | (0.0) | 40 | (0.0) | 47 | (0.0) | 29 | (0.0) | 16 | (0.0) | 23 | (0.0) | 14 | (0.0) |
| Parity | ||||||||||||||||
| 0 | 306,085 | (42.9) | 38,527 | (43.1) | 43,770 | (42.7) | 45,903 | (42.6) | 44,576 | (42.7) | 45,179 | (43.2) | 44,202 | (42.9) | 43,928 | (43.3) |
| ≥1 | 402,892 | (56.5) | 49,654 | (55.5) | 57,305 | (55.9) | 60,709 | (56.3) | 59,674 | (57.2) | 59,329 | (56.7) | 58,675 | (57.0) | 57,546 | (56.7) |
| Missing | 4144 | (0.6) | 1256 | (1.4) | 1408 | (1.4) | 1265 | (1.2) | 64 | (0.1) | 48 | (0.0) | 60 | (0.1) | 43 | (0.0) |
| Multiple birth | ||||||||||||||||
| Yes | 10,072 | (1.4) | 1278 | (1.4) | 1490 | (1.5) | 1553 | (1.4) | 1528 | (1.5) | 1423 | (1.4) | 1378 | (1.3) | 1422 | (1.4) |
| No | 703,049 | (98.6) | 88,159 | (98.6) | 100,993 | (98.5) | 106,324 | (98.6) | 102,786 | (98.5) | 103,133 | (98.6) | 101,559 | (98.7) | 100,095 | (98.6) |
| Country of birth | ||||||||||||||||
| Scandinavia | 467,815 | (65.6) | 60,828 | (68.0) | 67,377 | (65.7) | 69,920 | (64.8) | 67,965 | (65.2) | 68,250 | (65.3) | 67,212 | (65.3) | 66,263 | (65.3) |
| Outside Scandinavia | 179,445 | (25.2) | 18,114 | (20.3) | 22,380 | (21.8) | 27,908 | (25.9) | 27,816 | (26.7) | 28,306 | (27.1) | 28,165 | (27.4) | 26,756 | (26.4) |
| Missing | 65,861 | (9.2) | 10,495 | (11.7) | 12,726 | (12.4) | 10,049 | (9.3) | 8533 | (8.2) | 8000 | (7.7) | 7560 | (7.3) | 8498 | (8.4) |
| Maternal education status (y) | ||||||||||||||||
| ≤9 | 51,858 | (7.3) | 5755 | (6.4) | 6980 | (6.8) | 8559 | (7.9) | 8358 | (8.0) | 7991 | (7.6) | 7432 | (7.2) | 6783 | (6.7) |
| 10–12 | 224,867 | (31.5) | 27,970 | (31.3) | 32,128 | (31.3) | 34,644 | (32.1) | 33,884 | (32.5) | 33,877 | (32.4) | 32,363 | (31.4) | 30,001 | (29.6) |
| >12 | 312,802 | (43.9) | 36,877 | (41.2) | 41,699 | (40.7) | 46,019 | (42.7) | 46,105 | (44.2) | 47,504 | (45.4) | 46,928 | (45.6) | 47,670 | (47.0) |
| Missing | 123,594 | (17.3) | 18,835 | (21.1) | 21,676 | (21.2) | 18,655 | (17.3) | 15,967 | (15.3) | 15,184 | (14.5) | 16,214 | (15.8) | 17,063 | (16.8) |
| Season of conceptiona | ||||||||||||||||
| Winter | 186,013 | (26.1) | 21,807 | (24.4) | 26,372 | (25.7) | 28,483 | (26.4) | 27,433 | (26.3) | 27,811 | (26.6) | 27,198 | (26.4) | 26,909 | (26.5) |
| Spring | 189,348 | (26.6) | 25,970 | (29.0) | 26,713 | (26.1) | 28,191 | (26.1) | 27,303 | (26.2) | 27,457 | (26.3) | 27,164 | (26.4) | 26,550 | (26.2) |
| Summer | 170,177 | (23.9) | 23,824 | (26.6) | 24,751 | (24.2) | 25,388 | (23.5) | 24,377 | (23.4) | 24,336 | (23.3) | 23,824 | (23.1) | 23,677 | (23.3) |
| Fall | 167,583 | (23.5) | 17,836 | (19.9) | 24,647 | (24.0) | 25,815 | (23.9) | 25,201 | (24.2) | 24,952 | (23.9) | 24,751 | (24.0) | 24,381 | (24.0) |
| BMI (kg/m2) | ||||||||||||||||
| <18.5 | 17,126 | (2.4) | 2321 | (2.6) | 2555 | (2.5) | 2595 | (2.4) | 2361 | (2.3) | 2525 | (2.4) | 2479 | (2.4) | 2290 | (2.3) |
| 18.5–<25 | 376,453 | (52.8) | 50,470 | (56.4) | 56,329 | (55.0) | 57,239 | (53.1) | 52,041 | (49.9) | 54,522 | (52.1) | 53,377 | (51.9) | 52,475 | (51.7) |
| 25–<30 | 175,120 | (24.6) | 20,763 | (23.2) | 24,398 | (23.8) | 25,938 | (24.0) | 24,557 | (23.5) | 26,380 | (25.2) | 26,518 | (25.8) | 26,566 | (26.2) |
| 30–<35 | 67,469 | (9.5) | 7599 | (8.5) | 9042 | (8.8) | 9567 | (8.9) | 9682 | (9.3) | 10,446 | (10.0) | 10,386 | (10.1) | 10,747 | (10.6) |
| ≥35 | 29,541 | (4.1) | 3027 | (3.4) | 3826 | (3.7) | 4179 | (3.9) | 4225 | (4.1) | 4558 | (4.4) | 4749 | (4.6) | 4977 | (4.9) |
| Missing | 47,412 | (6.6) | 5257 | (5.9) | 6333 | (6.2) | 8359 | (7.7) | 11,448 | (11.0) | 6125 | (5.9) | 5428 | (5.3) | 4462 | (4.4) |
| Smoking in early pregnancy | ||||||||||||||||
| No | 655,643 | (91.9) | 83,360 | (0.0) | 95,519 | (93.2) | 99,123 | (91.9) | 90,388 | (86.6) | 97,014 | (92.8) | 95,835 | (93.1) | 94,404 | (93.0) |
| Yes | 30,331 | (4.3) | 4529 | (0.0) | 5031 | (4.9) | 4789 | (4.4) | 4272 | (4.1) | 4219 | (4.0) | 3874 | (3.8) | 3617 | (3.6) |
| Missing | 27,147 | (3.8) | 1548 | (0.0) | 1933 | (1.9) | 3965 | (3.7) | 9654 | (9.3) | 3323 | (3.2) | 3228 | (3.1) | 3496 | (3.4) |
BMI, body mass index.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Winter (December–February); Spring (March–May); Summer (June–August); Fall (September–November).
Supplemental Table 4.
Characteristics of births in Denmark by year, 2014 to 2020
| Characteristics | All |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| All births | 413,814 | 56,049 | 57,423 | 60,676 | 60,451 | 60,699 | 59,224 | 59,292 | ||||||||
| Gestational age (wk) | ||||||||||||||||
| Extremely preterm <28 | 1620 | (0.4) | 218 | (0.4) | 234 | (0.4) | 250 | (0.4) | 270 | (0.4) | 269 | (0.4) | 212 | (0.4) | 167 | (0.3) |
| Very preterm 28–<32 | 2393 | (0.6) | 329 | (0.6) | 335 | (0.6) | 366 | (0.6) | 354 | (0.6) | 352 | (0.6) | 336 | (0.6) | 321 | (0.5) |
| Moderate or late preterm 32–<37 | 19,411 | (4.7) | 2707 | (4.8) | 2717 | (4.7) | 2889 | (4.8) | 2847 | (4.7) | 2816 | (4.6) | 2719 | (4.6) | 2716 | (4.6) |
| Term 37–<42 | 381,218 | (92.1) | 51,684 | (92.2) | 52,908 | (92.1) | 55,774 | (91.9) | 55,616 | (92.0) | 55,890 | (92.1) | 54,565 | (92.1) | 54,781 | (92.4) |
| Postterm ≥42 | 9172 | (2.2) | 1111 | (2.0) | 1229 | (2.1) | 1397 | (2.3) | 1364 | (2.3) | 1372 | (2.3) | 1392 | (2.4) | 1307 | (2.2) |
| Maternal age (y) | ||||||||||||||||
| <20 | 3296 | (0.8) | 629 | (1.1) | 583 | (1.0) | 604 | (1.0) | 480 | (0.8) | 407 | (0.7) | 325 | (0.5) | 268 | (0.5) |
| 20–24 | 41,652 | (10.1) | 6255 | (11.2) | 6325 | (11.0) | 6631 | (10.9) | 6522 | (10.8) | 6008 | (9.9) | 5320 | (9.0) | 4591 | (7.7) |
| 25–29 | 138,920 | (33.6) | 17,965 | (32.1) | 18,813 | (32.8) | 20,383 | (33.6) | 20,515 | (33.9) | 20,670 | (34.1) | 20,311 | (34.3) | 20,263 | (34.2) |
| 30–34 | 144,304 | (34.9) | 19,083 | (34.0) | 19,575 | (34.1) | 20,455 | (33.7) | 20,502 | (33.9) | 21,231 | (35.0) | 21,329 | (36.0) | 22,129 | (37.3) |
| 35–39 | 69,390 | (16.8) | 9967 | (17.8) | 9924 | (17.3) | 10,302 | (17.0) | 10,013 | (16.6) | 9940 | (16.4) | 9560 | (16.1) | 9684 | (16.3) |
| ≥40 | 16,252 | (3.9) | 2150 | (3.8) | 2203 | (3.8) | 2301 | (3.8) | 2419 | (4.0) | 2443 | (4.0) | 2379 | (4.0) | 2357 | (4.0) |
| Missing | ||||||||||||||||
| Parity | ||||||||||||||||
| 0 | 190,650 | (46.1) | 25,247 | (45.0) | 26,081 | (45.4) | 28,315 | (46.7) | 28,222 | (46.7) | 28,289 | (46.6) | 27,473 | (46.4) | 27,023 | (45.6) |
| ≥1 | 223,120 | (53.9) | 30,802 | (55.0) | 31,342 | (54.6) | 32,361 | (53.3) | 32,229 | (53.3) | 32,410 | (53.4) | 31,751 | (53.6) | 32,225 | (54.3) |
| Missing | 44 | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | 44 | (0.1) | |||||||
| Multiple birth | ||||||||||||||||
| Yes | 6768 | (1.6) | 1066 | (1.9) | 972 | (1.7) | 1039 | (1.7) | 1044 | (1.7) | 921 | (1.5) | 881 | (1.5) | 845 | (1.4) |
| No | 407,046 | (98.4) | 54,983 | (98.1) | 56,451 | (98.3) | 59,637 | (98.3) | 59,407 | (98.3) | 59,778 | (98.5) | 58,343 | (98.5) | 58,447 | (98.6) |
| Season of conceptiona | ||||||||||||||||
| Winter | 105,919 | (25.6) | 14,290 | (25.5) | 15,063 | (26.2) | 15,752 | (26.0) | 15,514 | (25.7) | 15,146 | (25.0) | 15,170 | (25.6) | 14,984 | (25.3) |
| Spring | 97,751 | (23.6) | 13,287 | (23.7) | 13,644 | (23.8) | 14,062 | (23.2) | 14,454 | (23.9) | 14,401 | (23.7) | 13,959 | (23.6) | 13,944 | (23.5) |
| Summer | 100,506 | (24.3) | 13,648 | (24.4) | 13,536 | (23.6) | 14,726 | (24.3) | 14,750 | (24.4) | 15,176 | (25.0) | 14,261 | (24.1) | 14,409 | (24.3) |
| Fall | 109,638 | (26.5) | 14,824 | (26.4) | 15,180 | (26.4) | 16,136 | (26.6) | 15,733 | (26.0) | 15,976 | (26.3) | 15,834 | (26.7) | 15,955 | (26.9) |
| BMI (kg/m2) | ||||||||||||||||
| <18.5 | 17,330 | (0.6) | 2479 | (4.4) | 2611 | (4.5) | 2631 | (4.3) | 2577 | (4.3) | 2431 | (4.0) | 2265 | (3.8) | 2336 | (3.9) |
| 18.5–<25 | 233,608 | (7.8) | 32,283 | (57.6) | 32,871 | (57.2) | 33,978 | (56.0) | 32,866 | (54.4) | 32,916 | (54.2) | 34,450 | (58.2) | 34,244 | (57.8) |
| 25–<30 | 96,071 | (3.1) | 12,960 | (23.1) | 13,400 | (23.3) | 14,120 | (23.3) | 14,271 | (23.6) | 14,660 | (24.2) | 13,218 | (22.3) | 13,442 | (22.7) |
| 30–<35 | 37,343 | (1.2) | 4855 | (8.7) | 4961 | (8.6) | 5370 | (8.9) | 5550 | (9.2) | 5566 | (9.2) | 5532 | (9.3) | 5509 | (9.3) |
| ≥35 | 25,747 | (0.6) | 2619 | (4.7) | 2694 | (4.7) | 2942 | (4.8) | 3149 | (5.2) | 3302 | (5.4) | 5532 | (9.3) | 5509 | (9.3) |
| Missing | 8589 | (0.2) | 853 | (1.5) | 886 | (1.5) | 1635 | (2.7) | 2038 | (3.4) | 1824 | (3.0) | 760 | (1.3) | 593 | (1.0) |
| Smoking in early pregnancy | ||||||||||||||||
| No | 359,664 | (86.9) | 49,311 | (88.0) | 50,738 | (88.4) | 53,143 | (87.6) | 52,267 | (86.5) | 53,906 | (88.8) | 47,160 | (79.6) | 53,139 | (89.6) |
| Yes | 37,978 | (9.2) | 6257 | (11.2) | 6232 | (10.9) | 5997 | (9.9) | 5537 | (9.2) | 5028 | (8.3) | 4333 | (7.3) | 4594 | (7.7) |
| Missing | 16,172 | (3.9) | 481 | (0.9) | 453 | (0.8) | 1536 | (2.5) | 2647 | (4.4) | 1765 | (2.9) | 7731 | (13.1) | 1559 | (2.6) |
BMI, body mass index.
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Winter (December–February); Spring (March–May); Summer (June–August); Fall (September–November).
Supplemental Table 5.
Births and events included in difference-in-differences analysis, Norway
| Events | 2020 |
2014–2019 |
||||||
|---|---|---|---|---|---|---|---|---|
| After March 12 |
Before March 12 |
After March 12 |
Before March 12 |
|||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| 16 wk | ||||||||
| All births | 16,879 | 14,960 | 111,027 | 91,651 | ||||
| Preterm birth | 919 | (5.4) | 931 | (6.2) | 6181 | (5.6) | 5466 | (6.0) |
| Medically- indicated | 417 | (2.5) | 416 | (2.8) | 2571 | (2.3) | 2263 | (2.5) |
| Spontaneous | 502 | (3.0) | 515 | (3.4) | 3610 | (3.3) | 3203 | (3.5) |
| Very preterm birth | 156 | (0.9) | 157 | (1.0) | 996 | (0.9) | 918 | (1.0) |
| 12 wk | ||||||||
| All births | 12,487 | 11,488 | 82,158 | 72,844 | ||||
| Preterm birth | 685 | (5.5) | 723 | (6.3) | 4580 | (5.6) | 4274 | (5.9) |
| Medically indicated | 316 | (2.5) | 326 | (2.8) | 1931 | (2.4) | 1769 | (2.4) |
| Spontaneous | 369 | (3.0) | 397 | (3.5) | 2649 | (3.2) | 2505 | (3.4) |
| Very preterm birth | 122 | (1.0) | 115 | (1.0) | 776 | (0.9) | 710 | (1.0) |
| 8 wk | ||||||||
| All births | 8190 | 7971 | 54,359 | 51,521 | ||||
| Preterm birth | 457 | (5.6) | 487 | (6.1) | 3032 | (5.6) | 2879 | (5.6) |
| Medically- indicated | 218 | (2.7) | 230 | (2.9) | 1287 | (2.4) | 1194 | (2.3) |
| Spontaneous | 239 | (2.9) | 257 | (3.2) | 1745 | (3.2) | 1685 | (3.3) |
| Very preterm birth | 70 | (0.9) | 84 | (1.1) | 533 | (1.0) | 480 | (0.9) |
| 4 wk | ||||||||
| All births | 3939 | 4080 | 26,440 | 25,668 | ||||
| Preterm birth | 216 | (5.5) | 231 | (5.7) | 1493 | (5.6) | 1473 | (5.7) |
| Medically- indicated | 95 | (2.4) | 97 | (2.4) | 650 | (2.5) | 611 | (2.4) |
| Spontaneous | 121 | (3.1) | 134 | (3.3) | 843 | (3.2) | 862 | (3.4) |
| Very preterm birth | 39 | (1.0) | 42 | (1.0) | 263 | (1.0) | 245 | (1.0) |
| 2 wk | ||||||||
| All births | 1941 | 2065 | 12,964 | 12,596 | ||||
| Preterm birth | 111 | (5.7) | 100 | (4.8) | 739 | (5.7) | 687 | (5.5) |
| Medically- indicated | 48 | (2.5) | 39 | (1.9) | 311 | (2.4) | 272 | (2.2) |
| Spontaneous | 63 | (3.2) | 61 | (3.0) | 428 | (3.3) | 415 | (3.3) |
| Very preterm birth | 20 | (1.0) | 17 | (0.8) | 128 | (1.0) | 116 | (0.9) |
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Supplemental Table 6.
Births and events included in difference-in-differences analysis, Sweden
| Events | 2020 |
2014–2019 |
||||||
|---|---|---|---|---|---|---|---|---|
| After March 12 |
Before March 12 |
After March 12 |
Before March 12 |
|||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| 16 wk | ||||||||
| All births | 32,693 | 29,656 | 195,802 | 163,393 | ||||
| Preterm birth | 1728 | (5.3) | 1708 | (5.8) | 10,866 | (5.5) | 9725 | (6.0) |
| Medically indicated | 591 | (1.8) | 592 | (2.0) | 3400 | (1.7) | 3050 | (1.9) |
| Spontaneous | 1137 | (3.5) | 1116 | (3.8) | 7466 | (3.8) | 6675 | (4.1) |
| Very preterm birth | 269 | (0.8) | 293 | (1.0) | 1798 | (0.9) | 1642 | (1.0) |
| 12 wk | ||||||||
| All births | 24,477 | 22,869 | 146,034 | 128,528 | ||||
| Preterm birth | 1298 | (5.3) | 1284 | (5.6) | 8110 | (5.6) | 7562 | (5.9) |
| Medically indicated | 439 | (1.8) | 447 | (2.0) | 2546 | (1.7) | 2338 | (1.8) |
| Spontaneous | 859 | (3.5) | 837 | (3.7) | 5564 | (3.8) | 5224 | (4.1) |
| Very preterm birth | 210 | (0.9) | 222 | (1.0) | 1368 | (0.9) | 1277 | (1.0) |
| 8 wk | ||||||||
| All births | 16,108 | 15,732 | 96,458 | 90,502 | ||||
| Preterm birth | 890 | (5.5) | 859 | (5.5) | 5399 | (5.6) | 5125 | (5.7) |
| Medically- indicated | 315 | (2.0) | 310 | (2.0) | 1741 | (1.8) | 1609 | (1.8) |
| Spontaneous | 575 | (3.6) | 549 | (3.5) | 3658 | (3.8) | 3516 | (3.9) |
| Very preterm birth | 134 | (0.8) | 144 | (0.9) | 920 | (1.0) | 885 | (1.0) |
| 4 wk | ||||||||
| All births | 7868 | 7876 | 47,134 | 45,895 | ||||
| Preterm birth | 454 | (5.8) | 451 | (5.7) | 2636 | (5.6) | 2548 | (5.6) |
| Medically- indicated | 145 | (1.8) | 164 | (2.1) | 852 | (1.8) | 798 | (1.7) |
| Spontaneous | 309 | (3.9) | 287 | (3.6) | 1784 | (3.8) | 1750 | (3.8) |
| Very preterm birth | 58 | (0.7) | 79 | (1.0) | 442 | (0.9) | 461 | (1.0) |
| 2 wk | ||||||||
| All births | 3937 | 3987 | 23,386 | 23,135 | ||||
| Preterm birth | 223 | (5.7) | 210 | (5.3) | 1318 | (5.6) | 1287 | (5.6) |
| Medically- indicated | 78 | (2.0) | 79 | (2.0) | 438 | (1.9) | 400 | (1.7) |
| Spontaneous | 145 | (3.7) | 131 | (3.3) | 880 | (3.8) | 887 | (3.8) |
| Very preterm birth | 35 | (0.9) | 41 | (1.0) | 220 | (0.9) | 227 | (1.0) |
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
Supplemental Table 7.
Births and events included in difference-in-differences analysis, Denmark
| Events | 2020 |
2014–2019 |
||||||
|---|---|---|---|---|---|---|---|---|
| After March 12 |
Before March 12 |
After March 12 |
Before March 12 |
|||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| 16 wk | ||||||||
| All births | 18,152 | 18,272 | 107,551 | 95,909 | ||||
| Preterm birth | 1017 | (5.6) | 1043 | (5.7) | 6290 | (5.8) | 5640 | (5.9) |
| Medically indicated | 329 | (1.8) | 346 | (1.9) | 2153 | (2.0) | 1913 | (2.0) |
| Spontaneous | 688 | (3.8) | 697 | (3.8) | 4137 | (3.8) | 3727 | (3.9) |
| Very preterm birth | 161 | (0.9) | 165 | (0.9) | 1101 | (1.0) | 1024 | (1.1) |
| 12 wk | ||||||||
| All births | 13,214 | 13,470 | 79,414 | 74,718 | ||||
| Preterm birth | 733 | (5.5) | 771 | (5.7) | 4636 | (5.8) | 4364 | (5.8) |
| Medically indicated | 246 | (1.9) | 257 | (1.9) | 1586 | (2.0) | 1508 | (2.0) |
| Spontaneous | 487 | (3.7) | 514 | (3.8) | 3050 | (3.8) | 2856 | (3.8) |
| Very preterm birth | 115 | (0.9) | 114 | (0.8) | 816 | (1.0) | 791 | (1.1) |
| 8 wk | ||||||||
| All births | 8664 | 8894 | 52,167 | 51,853 | ||||
| Preterm birth | 482 | (5.6) | 493 | (5.5) | 3039 | (5.8) | 2962 | (5.7) |
| Medically- indicated | 161 | (1.9) | 172 | (1.9) | 1047 | (2.0) | 997 | (1.9) |
| Spontaneous | 321 | (3.7) | 321 | (3.6) | 1992 | (3.8) | 1965 | (3.8) |
| Very preterm birth | 76 | (0.9) | 68 | (0.8) | 523 | (1.0) | 539 | (1.0) |
| 4 wk | ||||||||
| All births | 4207 | 4469 | 25,828 | 26,236 | ||||
| Preterm birth | 228 | (5.4) | 232 | (5.2) | 1495 | (5.8) | 1506 | (5.7) |
| Medically- indicated | 70 | (1.7) | 89 | (2.0) | 502 | (1.9) | 518 | (2.0) |
| Spontaneous | 158 | (3.8) | 143 | (3.2) | 993 | (3.8) | 988 | (3.8) |
| Very preterm birth | 35 | (0.8) | 29 | (0.6) | 275 | (1.1) | 288 | (1.1) |
| 2 wk | ||||||||
| All births | 2101 | 2269 | 12,923 | 12,984 | ||||
| Preterm birth | 108 | (5.1) | 109 | (4.8) | 733 | (5.7) | 737 | (5.7) |
| Medically- indicated | 39 | (1.9) | 34 | (1.5) | 245 | (1.9) | 243 | (1.9) |
| Spontaneous | 69 | (3.3) | 75 | (3.3) | 488 | (3.8) | 494 | (3.8) |
| Very preterm birth | 17 | (0.8) | 20 | (0.9) | 128 | (1.0) | 151 | (1.2) |
Oakley et al. Preterm birth and COVID-19 mitigation measures in Scandinavia. Am J Obstet Gynecol 2022.
References
- 1.Naurin E., Markstedt E., Stolle D., et al. Pregnant under the pressure of a pandemic: a large-scale longitudinal survey before and during the COVID-19 outbreak. Eur J Public Health. 2021;31:7–13. doi: 10.1093/eurpub/ckaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perera F., Berberian A., Cooley D., et al. Potential health benefits of sustained air quality improvements in New York City: a simulation based on air pollution levels during the COVID-19 shutdown. Environ Res. 2021;193:110555. doi: 10.1016/j.envres.2020.110555. [DOI] [PubMed] [Google Scholar]
- 3.Philip R.K., Purtill H., Reidy E., et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessami K., Romanelli C., Chiurazzi M., Cozzolino M. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1843155. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Lebel C., MacKinnon A., Bagshawe M., Tomfohr-Madsen L., Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. 2020;277:5–13. doi: 10.1016/j.jad.2020.07.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceulemans M., Foulon V., Ngo E., et al. Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic-a multinational cross-sectional study. Acta Obstet Gynecol Scand. 2021;100:1219–1229. doi: 10.1111/aogs.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townsend R., Chmielewska B., Barratt I., et al. Global changes in maternity care provision during the COVID-19 pandemic: a systematic review and meta-analysis. EClinicalMedicine. 2021;37:100947. doi: 10.1016/j.eclinm.2021.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chmielewska B., Barratt I., Townsend R., et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strand L.B., Barnett A.G., Tong S. The influence of season and ambient temperature on birth outcomes: a review of the epidemiological literature. Environ Res. 2011;111:451–462. doi: 10.1016/j.envres.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg R.L., McClure E.M. Have coronavirus disease 2019 (COVID-19) community lockdowns reduced preterm birth rates? Obstet Gynecol. 2021;137:399–402. doi: 10.1097/AOG.0000000000004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludvigsson J.F. The first eight months of Sweden’s COVID-19 strategy and the key actions and actors that were involved. Acta Paediatr. 2020;109:2459–2471. doi: 10.1111/apa.15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mens H., Koch A., Chaine M., Andersen A.B. The hammer vs mitigation-a comparative retrospective register study of the Swedish and Danish national responses to the COVID-19 pandemic in 2020. APMIS. 2021;129:384–392. doi: 10.1111/apm.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarmol-Matusiak E.A., Cipriano L.E., Stranges S. A comparison of COVID-19 epidemiological indicators in Sweden, Norway, Denmark, and Finland. Scand J Public Health. 2021;49:69–78. doi: 10.1177/1403494820980264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. Coronavirus pandemic (COVID-19) 2020. https://ourworldindata.org/coronavirus Available at:
- 15.Zhang L., Brikell I., Dalsgaard S., Chang Z. Public mobility and social media attention in response to COVID-19 in Sweden and Denmark. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.33478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irgens L.M. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–439. [PubMed] [Google Scholar]
- 17.Stephansson O., Petersson K., Björk C., Conner P., Wikström A.K. The Swedish Pregnancy Register - for quality of care improvement and research. Acta Obstet Gynecol Scand. 2018;97:466–476. doi: 10.1111/aogs.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bliddal M., Broe A., Pottegård A., Olsen J., Langhoff-Roos J. The Danish medical birth register. Eur J Epidemiol. 2018;33:27–36. doi: 10.1007/s10654-018-0356-1. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M., Schmidt S.A.J., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen C.B., Gøtzsche H., Møller J.O., Mortensen P.B. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 21.Dimick J.B., Ryan A.M. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312:2401–2402. doi: 10.1001/jama.2014.16153. [DOI] [PubMed] [Google Scholar]
- 22.Wing C., Simon K., Bello-Gomez R.A. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health. 2018;39:453–469. doi: 10.1146/annurev-publhealth-040617-013507. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Been J.V., Burgos Ochoa L., Bertens L.C.M., Schoenmakers S., Steegers E.A.P., Reiss I.K.M. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5:e604–e611. doi: 10.1016/S2468-2667(20)30223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berghella V., Boelig R., Roman A., Burd J., Anderson K. Decreased incidence of preterm birth during coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020;2:100258. doi: 10.1016/j.ajogmf.2020.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallo L.A., Gallo T.F., Borg D.J., Moritz K.M., Clifton V.L., Kumar S. A decline in planned, but not spontaneous, preterm birth rates in a large Australian tertiary maternity centre during COVID-19 mitigation measures. Aust N Z J Obstet Gynaecol. 2021 doi: 10.1111/ajo.13406. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedermann G., Hedley P.L., Bækvad-Hansen M., et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106:93–95. doi: 10.1136/archdischild-2020-319990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasuga Y., Tanaka M., Ochiai D. Preterm delivery and hypertensive disorder of pregnancy were reduced during the COVID-19 pandemic: a single hospital-based study. J Obstet Gynaecol Res. 2020 doi: 10.1111/jog.14518. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemon L., Edwards R.P., Simhan H.N. What is driving the decreased incidence of preterm birth during the coronavirus disease 2019 pandemic? Am J Obstet Gynecol MFM. 2021;3:100330. doi: 10.1016/j.ajogmf.2021.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matheson A., McGannon C.J., Malhotra A., et al. Prematurity rates during the coronavirus disease 2019 (COVID-19) pandemic lockdown in Melbourne, Australia. Obstet Gynecol. 2021;137:405–407. doi: 10.1097/AOG.0000000000004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer R., Bart Y., Tsur A., et al. A marked decrease in preterm deliveries during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol. 2021;224:234–237. doi: 10.1016/j.ajog.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Curtis M., Villani L., Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106:456. doi: 10.1136/archdischild-2020-320682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson A.N., Snelgrove J.W., Sutradhar R., Everett K., Liu N., Baxter N.N. Perinatal outcomes during the COVID-19 pandemic in Ontario, Canada. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gemmill A, Casey JA, Catalano R, Karasek D, Bruckner T. Changes in live births, preterm birth, low birth weight, and cesarean deliveries in the United States during the SARS-CoV-2 pandemic. medRxiv. Preprint posted online March 25, 2021. https://doi.org/10.1101/2021.03.20.21253990
- 35.Rolnik D.L., Matheson A., Liu Y., et al. Impact of COVID-19 pandemic restrictions on pregnancy duration and outcome in Melbourne, Australia. Ultrasound Obstet Gynecol. 2021;58:677–687. doi: 10.1002/uog.23743. [DOI] [PubMed] [Google Scholar]
- 36.Einarsdóttir K., Swift E.M., Zoega H. Changes in obstetric interventions and preterm birth during COVID-19: a nationwide study from Iceland. Acta Obstet Gynecol Scand. 2021;100:1924–1930. doi: 10.1111/aogs.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handley S.C., Mullin A.M., Elovitz M.A., et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, March-June 2020. JAMA. 2021;325:87–89. doi: 10.1001/jama.2020.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalil A., von Dadelszen P., Draycott T., Ugwumadu A., O’Brien P., Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Main E.K., Chang S.C., Carpenter A.M., et al. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021;224:239–241. doi: 10.1016/j.ajog.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter F., Strasser A.S., Suarez-Farinas M., et al. Neonatal outcomes during the COVID-19 pandemic in New York City. Pediatr Res. 2021 doi: 10.1038/s41390-021-01513-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood R., Sinnott C., Goldfarb I., Clapp M., McElrath T., Little S. Preterm birth during the coronavirus disease 2019 (COVID-19) pandemic in a large hospital system in the United States. Obstet Gynecol. 2021;137:403–404. doi: 10.1097/AOG.0000000000004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasternak B., Neovius M., Söderling J., et al. Preterm birth and stillbirth during the COVID-19 pandemic in Sweden: a nationwide cohort study. Ann Intern Med. 2021;174:873–875. doi: 10.7326/M20-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunes I.S., Vrangbæk K., Byrkjeflot H., et al. Nordic responses to Covid-19: governance and policy measures in the early phases of the pandemic. Health Policy. 2021 doi: 10.1016/j.healthpol.2021.08.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helsingen L.M., Refsum E., Gjøstein D.K., et al. The COVID-19 pandemic in Norway and Sweden - threats, trust, and impact on daily life: a comparative survey. BMC Public Health. 2020;20:1597. doi: 10.1186/s12889-020-09615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emborg H.-D., Carnahan A., Bragstad K., et al. Abrupt termination of the 2019/20 influenza season following preventive measures against COVID-19 in Denmark, Norway and Sweden. Euro Surveill. 2021;26:2001160. doi: 10.2807/1560-7917.ES.2021.26.22.2001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodilsen J., Nielsen P.B., Søgaard M., et al. Hospital admission and mortality rates for non-covid diseases in Denmark during covid-19 pandemic: nationwide population based cohort study. BMJ. 2021;373:n1135. doi: 10.1136/bmj.n1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cobo T., Kacerovsky M., Jacobsson B. Risk factors for spontaneous preterm delivery. Int J Gynaecol Obstet. 2020;150:17–23. doi: 10.1002/ijgo.13184. [DOI] [PubMed] [Google Scholar]
- 48.Chang H.H., Larson J., Blencowe H., et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381:223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norman M., Navér L., Söderling J., et al. Association of maternal SARS-CoV-2 infection in pregnancy with neonatal outcomes. JAMA. 2021;325:2076–2086. doi: 10.1001/jama.2021.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]