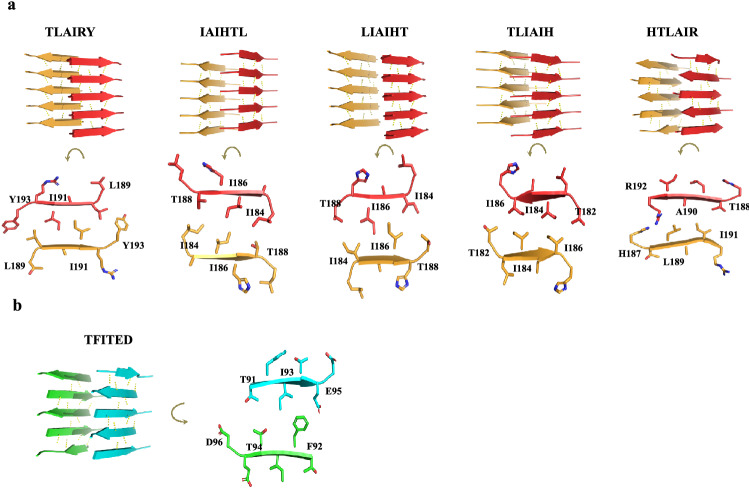
Figure 3.
Hexapeptides derived from P10 and P4 (only a single stretch) form steric zippers in silico. P10 (a) and P4 (b) sequences were analyzed by Cordax (LOURO et al., 2020). The algorithm identified five hexapeptides in the sequence of P10 (TLIAIH, LIAIHT, IAIHTL, TLAIRY and HTLAIR) capable of forming steric zippers. Except for HTLAIR, each peptide forms two sheets (orange and red) composed of parallel β-strands (antiparallel in HTLAIR). In panel (a), the lower images show details of the interdigitation of the lateral chains of the amino acids in the steric zippers facing the interior of the bilayer. (b) Only one hexapeptide of P4 (TFITED; blue and green) adopts a steric zipper structure in an antiparallel fashion.