Fig. 1.
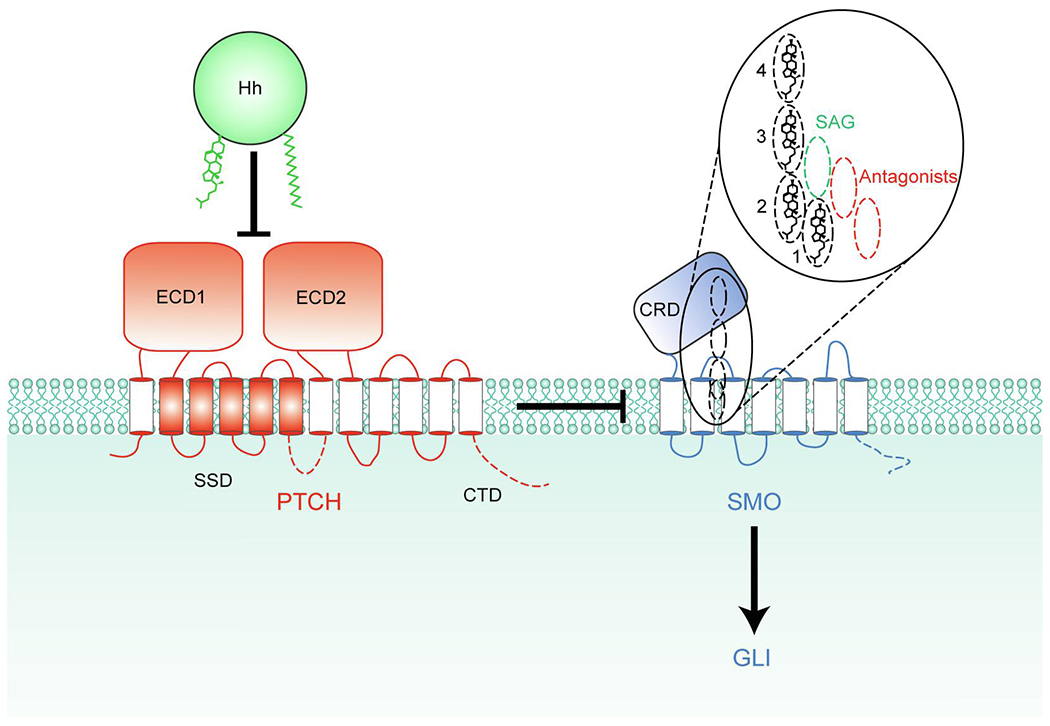
PTCH inhibits SMO by an unknown mechanism. Binding of Hh inhibits PTCH, allowing SMO activation. The 4 partially overlapping sites within SMO that can be occupied by sterols are depicted as dashed ovals, and their vertical positions compared to the synthetic SMO agonist (SAG) and various antagonists are shown in the inset. Sterols in all 4 sites within SMO have the same orientation (hydroxyl group facing towards the extracellular side and the side chain facing towards the intracellular side) potentially allowing sterols to move between these sites without flipping. The intracellular domains of PTCH1 and SMO missing from the structures, including the C-terminal domain (CTD) of PTCH1, are indicated by dashed lines. ECD1 and ECD2, extracellular domains 1 and 2; SSD, sterol-sensing domain; CRD, cysteine-rich domain.
