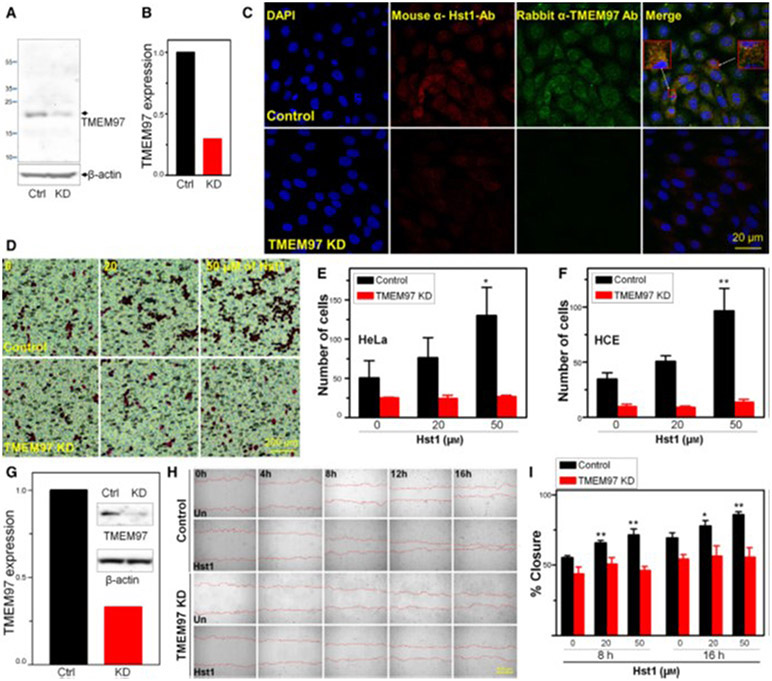
Fig. 4.
Knockdown (KD) of TMEM97 inhibits Hst1 induced migration and wound healing. (A) HCE cells were transfected for 48h with either control siRNA (Ctrl) or TMEM97 siRNA [knockdown (KD)]. Whole cell lysates were then subjected to western blotting with antibodies against TMEM97. (B) Densitometry measurements indicate that TMEM97KD reduced protein levels by 70%. Notable is the significant reduction in TMEM97 signal in the TMEM97 siRNA treated samples indicating successful KD. (C) Immunolocalization shows internalization of Hst1 to HCE and colocalization with TMEM97 in the perinuclear/ER area with control siRNA transfection and loss of both TMEM97 signal and loss of internalized Hst1 signal in KD cells, suggesting that TMEM97 is either necessary for internalization or localization of Hst1. Scale bar = 20 μm. Images are representative of three separate experiments. (D–F) Boyden chamber migration assay testing for response of HeLa and HCE cells to migrate across a membrane toward FBS in response to treatment with Hst1. Control siRNA transfected cells increase transmigration in a dose-dependent manner in response to Hst1 exposure. This responsiveness to Hst1 is lost in TMEM97 KD cells, highlighting the importance of TMEM97 for this action of Hst1. (D) Images of Boyden chamber membranes showing HeLa cells stained with Eosin. Scale bar = 200μm. (E–F) Cell counting of transmigrated cells (E = HeLa, F = HCE) in the Boyden chamber assay with and without KD of TMEM97 over a range of concentrations of Hst1. Notable is the loss of Hst1 responsiveness in both cell types after KD of TMEM97. Statistical significance was determined by 2-way ANOVA with Bonferroni’s post hoc test. **P < 0.01. Error bars indicate Standard Error of the Mean. Experiments were performed in triplicate. Statistical analyses were performed using GraphPad prism software 5.0 (GraphPad Software, La Jolla, CA, USA). (G–I) Wound healing experiment testing necessity of TMEM97 for Hst1 responsiveness. (G) shRNA mediated KD of TMEM97 in HCE cells was tested using western blotting for TMEM97 protein comparing control and KD cells. Densitometry measurements indicate that shRNA KD reduced protein levels of TMEM97 by 70%. (H) Time lapse microscopy [Image Express Micro (Molecular Devices, CA, USA)] at 4x magnification, after standardized wounding of confluent HCE cells in serum free conditions, was used to quantify migration rates and scratch closure times with and without Hst1 cotreatment at the time of wounding, with and without TMEM97 KD. Scale bar = 500μm. (I) Bar graph depicting scratch closure % over time. Notably, we found a statistically significant improvement in scratch closure rates (versus untreated control) with Hst1 treatment (20 or 50 μm) of concentrations at 8 and 16 h and loss of this response to Hst1 application in the TMEM97 KD cells. Statistical significance was determined by 1-way ANOVA with Bonferroni’s post hoc test. *P < 0.05; **P < 0.01. Error bars indicate Standard Error of the Mean. Experiments were performed in triplicate. Statistical analyses were performed using GraphPad prism software 5.0 (GraphPad Software, La Jolla, CA, USA). Taken together these results suggest that KD of TMEM97 can cause loss of Hst1 internalization or localization in HCE cells. Moreover, TMEM97 appears to be necessary for increases in HCE and HeLa cell migration and HCE scratch closure in response to Hst1.