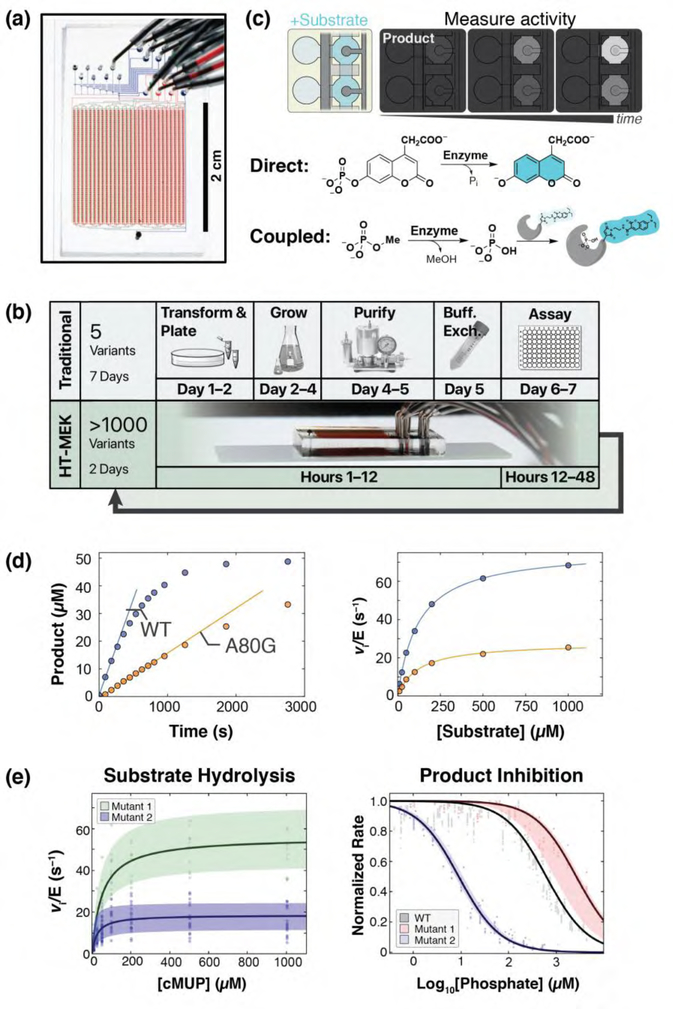
Figure 2. Application of HT-MEK to study catalysis and inhibition.
(a) HT-MEK uses a valved microfluidic device containing >1500 chambers to (b) in vitro express, purify, and assay 1500 enzyme variants in days. Iteratively varying substrates, inhibitors, and conditions give kinetic and thermodynamic parameters of function. (c) Reactions are performed by introducing fluorogenic substrate (light blue) into reaction chambers and synchronously exposing surface-immobilized enzyme to the substrate with pneumatic valving. Product (darker blue) is quantified by fluorescence over time, using direct (fluorogenic) or indirect (coupled) assays. The assays shown are for phosphatase activity or for any enzyme that directly or indirectly generates inorganic phosphate. (d) Example on-chip fluorogenic substrate turnover curves for the PafA phosphatase with fit initial rates (left) and Michaelis-Menten curves (right) for WT (blue) and mutant (orange) PafA variants. A per-chamber standard curve is used to convert fluorescence to product concentration. (e) Many replicates (chambers) for each variant (Mutant 1: Y74V, Mutant 2: Y112G) over multiple chips are used to calculate bootstrap errors on fit Michaelis-Menten (left) and competitive inhibition (right) parameters (reproduced from Markin, Mokhtari, et al. (2021) [47]).