Figure 2. Estradiol (estrogen) regulates 5‐HTR2B (5‐HTR2 subtype B) gene expression and protein levels in HL‐1 and adult primary mouse cardiomyocytes.
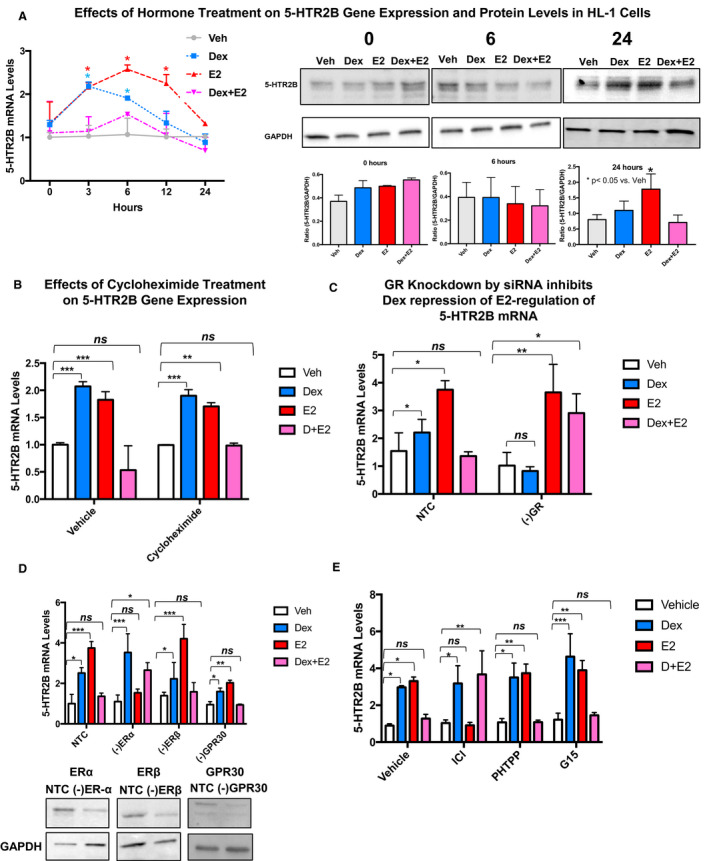
A, HL‐1 cells were treated with vehicle (Veh; gray line), 100 nmol/L dexamethasone (Dex; blue), estradiol (estrogen [E2]; red), and dexamethasone+estrogen (Dex+E2; pink) for the indicated times (0–24 hours), and 5‐HTR2B levels were analyzed by quantitative real‐time polymerase chain reaction and Western blotting. Estrogen steadily increased the mRNA expression of 5‐HTR2B in the time course experiment from 3 to 12 hours. Dexamethasone led to 5‐HTR2B increased expression at the 3‐hour time point. Dexamethasone+estrogen prevented estrogen‐induced upregulation of 5‐HTR2B expression at all time points. No significant changes in protein levels were observed at baseline (0 hours) or 6 hours following hormone treatment. The 5‐HTR2B protein levels were significantly increased in response to estrogen at 24 hours. Cotreatment of HL‐1 cells with dexamethasone+estrogen abolished estrogen effects on 5‐HTR2B at the protein level. Levels of 5‐HTR2B were normalized to the loading control GAPDH. B, HL‐1 cells were pretreated for 3 hours with vehicle (control) or 10 μg/mL of cycloheximide. Then cells were exposed to 100 nmol/L dexamethasone, 10 nmol/L estrogen, or dexamethasone+estrogen for 6 hours. Cycloheximide treatment did not have significant effects on 5‐HTR2B regulation by estrogen, suggesting that 5‐HTR2B is a direct target of estrogen. C, HL‐1 cells were transfected with NTC siRNA or glucocorticoid receptor (GR) siRNA (‐GR). At 48 hours after transfection, cells were treated with vehicle, 100 nmol/L dexamethasone, 10 nmol/L estrogen, or dexamethasone+estrogen for 3 hours. Downregulation of GR levels significantly blocked dexamethasone effects on 5‐HTR2B mRNA levels, showing that GR is required for dexamethasone effects. D, HL‐1 cells were also transfected with ERα (estrogen receptor α) siRNA (‐ERα), ERβ (‐ERβ) siRNA, and GPR30 (G protein‐coupled estrogen receptor 1) siRNA (‐GPR30) as described previously. Knocking down ERα abolished estrogen regulation of 5‐HTR2B, demonstrating that 5‐HTR2B regulation by estrogen is dependent on ERα expression. E, HL‐1 cells were treated with the following 3 estrogen receptor antagonists: ICI (182,780; fulvestrant; ERα antagonist), PHTPP (4‐[2‐Phenyl‐5,7‐bis(trifluoromethyl) pyrazolo[1,5‐a]pyrimidin‐3‐yl]phenol; ERβ antagonist), and G‐15 ([3aS,4R,9bR]‐4‐[6‐bromo‐1,3‐benzodioxol‐5‐yl]‐3a,4,5,9b‐tetrahydro‐3H‐cyclopenta[c]quinoline; G‐protein‐coupled estrogen receptor [GPER/GPR30] antagonist). After 1 hour of antagonist treatment, cells were subjected to hormone treatments. ICI significantly inhibited the estrogen upregulation of 5‐HTR2B. For the cycloheximide, siRNA, and antagonists experiments, 5‐HTR2B levels were measured by quantitative real‐time polymerase chain reaction. mRNA was normalized to cyclophillin B for all mRNA experiments. We used the same doses of hormones for all treatments. The figure includes representative Western blots confirming the changes in 5‐HTR2B protein levels and the knockdown of each individual receptor. Ordinary 1‐way ANOVA with Dunnett multiple comparisons analysis was used to evaluate differences among group treatments unless otherwise specified. Data represent the mean±SE (n=3–5 independent samples per group). *P<0.05; **P<0.01; ***P<0.001, ns=not significant.
