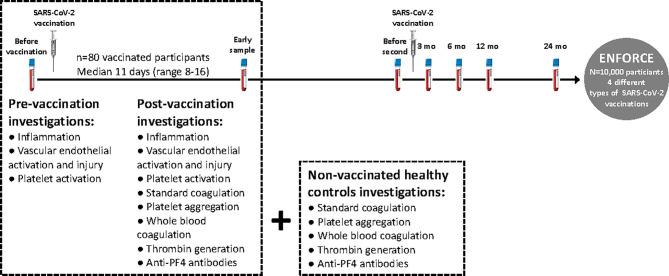
Figure 1.
Study design. Eighty participants (n=80) included in the Danish national vaccine trial ENFORCE, recently vaccinated with AZ or mRNA vaccines against COVID-19 were invited to provide an early blood sample post-vaccination (median of 11 days (range 8-16) post-vaccination). Pre-vaccination samples were available from all participants via the ENFORCE study. Since standard coagulation tests, platelet aggregation, whole blood coagulation (Thromboelastometry) and thrombin generation were not available at the pre-vaccination sample, non-vaccinated age and gender matched healthy individuals were invited to participate as controls.