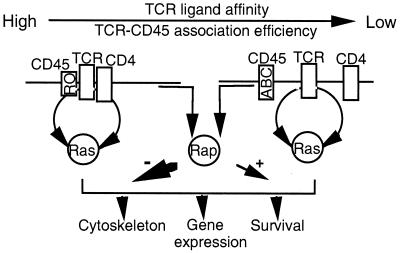
FIG. 7.
Model of the putative functional organization of Ras and Rap1 by the TCR-CD4-CD45 interacting complex. Low-molecular-weight isoforms of CD45 (RO) efficiently interact with the TCR, thereby increasing the amplitude of the TCR effector signals. Under these conditions, stimulation of the TCR-CD4 complex with the high-affinity ligand (e.g., MAb 3D3) delivers a strong signal which involves Rap1 primarily in the negative regulation of Ras effector functions. In contrast, stimulation of the TCR with intermediate-to-low-affinity ligands (e.g., MAb 5A) fails to recruit CD4 and, in the presence of the high-molecular-weight isoform of CD45 (ABC), generates a signal that is below the threshold required for the negative regulation of Ras by Rap1 yet is sufficient to positively engage Rap1 toward the Ras-MAP kinase signaling pathway.