Abstract
The aggressive and highly metastatic nature of triple‐negative breast cancer (TNBC) causes patients to suffer from the poor outcome. HIF‐1 signalling pathway is a prominent pathway that contributes to angiogenesis and metastasis progression in tumours. On the contrary, the undeniable importance of circular RNAs (circRNAs) as multifunctional non‐coding RNAs (ncRNAs) has been identified in breast cancer. These ncRNAs owing to their high stability and specificity have been becoming a hotspot in cancer researches. circRNAs act as competing endogenous RNAs (ceRNAs) and compete with mRNAs for shared miRNAs, thus modulate gene expression. Since the most dysregulated biological functions in TNBC are associated with cellular invasion, understanding the molecular pathogenesis of these processes is a crucial step towards the development of new treatment approaches. The purpose of this study is to undermine the circRNA‐associated ceRNA network involved in HIF‐1 signalling in TNBC using an integrative bioinformatics approach. In the next step, the novel circ_0047303‐mediated ceRNA regulatory axes have been extracted and validated across TNBC samples. We show that circ_0047303 has the highest degree in the circRNA‐associated ceRNA network and shows a significant up‐expression in TNBC. Moreover, our results suggest that circ_0047303 could mediate the upregulation of key angiogenesis‐related genes, including HIF‐1, EIF4E2 and VEGFA in TNBC through sponging the tumour‐suppressive miRNAs. The circ_0047303 could be a promising molecular biomarker and/or therapeutic target for TNBC.
Keywords: angiogenesis, ceRNA network, circ_0047303, HIF‐1 signalling, microarray, triple‐negative breast cancer
1. INTRODUCTION
About 15% of breast cancer (BC) patients are diagnosed with the triple‐negative subtype, which is characterized by an aggressive, highly metastatic entity 1 and demonstrates the least favourable outcome among all BC subtypes. In triple‐negative breast cancer (TNBC), none of the main receptors (oestrogen receptor and HER2) for BC targeted therapies are expressed, and chemotherapy remains the standard therapeutic choice. 2 Although TNBC has a high metastasis tendency and a poor outcome, targeted therapy is still limited. 3 Therefore, it is necessary to seek out potential approaches for targeted therapies. The emergence of high‐throughput techniques such as microarray was accompanied by the identification of BC expression profiles with potential application in diagnosis, prognosis and therapy. 4 , 5
In the majority of tumour cells, reduction of oxygen supply (hypoxia) is a common event that evokes a myriad of consequences in tumour cells, namely metastasis, vascularization, epithelial‐to‐mesenchymal transition (EMT) and resistance to therapeutic approaches. 6 Cellular responses to hypoxia are orchestrated by a group of transcription factors known as hypoxia‐inducible factors (HIFs). These factors trigger the expression of genes essential for adjustment to hypoxic conditions 7 and affect tumour‐related processes, including metastasis and chemoresistance. 8 For instance, HIF‐1α impacted metastasis in the MDA‐MB‐435 TNBC cell line. 9 Expression of HIF‐1α was associated with poor prognosis in BC patients. 10 In solid tumours, HIF‐1 binds to and upregulates VEGFA under hypoxic conditions. This effect, in turn, promotes the formation of new vessels in tumours. 11 Altogether, the HIF‐1 signalling pathway is a major driver of angiogenesis and metastasis progression. We previously showed that the most dysregulated biological functions in TNBC are the ones that are involved in proliferation and invasion. 12 Hence, as TNBC tumours are likely to metastasize, further investigation is needed to better clarify underlying molecular mechanisms in dysregulating HIF‐1 signalling pathway genes.
Years of cancer research provided a great body of evidence that indicates the role of microRNAs (miRNAs) and circular RNAs (circRNAs) in BC (For a complete list of these studies, please refer to [13]). These non‐coding RNAs interact with each other and orchestrate significant processes such as metastasis in cancer cells. 14 , 15 One of the functions of circRNAs is to sponge miRNAs, which in turn affects the expression of their target mRNAs. 16 Such interactions have been investigated in the context of competing endogenous RNA (ceRNA) regulatory networks in BC. 17 , 18
In the current study, firstly, we used an integrative approach to determine potential HIF‐1 signalling‐related circRNA‐miRNA‐mRNA axes involved in TNBC tumours using microarray datasets and a variety of bioinformatics tools. In the next step, we sought to underpin and experimentally validate the candidate key differentially expressed (DE) circ_0047303 along with its downstream target genes in triple‐negative tumour samples.
2. MATERIALS AND METHODS
2.1. Investigation of discriminative differentially expressed circRNAs (DECs) in triple‐negative breast cancer (TNBC) versus normal mammary gland
The microarray dataset GSE101124 related to circRNA expression profile in TNBC containing the data of four triple‐negative tumours and three normal mammary tissues was achieved from the NCBI Gene Expression Omnibus (GEO) database. 19 The data were normalized (Quantile Normalization) and log2‐transformed (Figure 1). Then to screen the DECs in TNBC in comparison with normal mammary tissues, the Limma package in R software was used. The transcripts with the criteria of |log2(foldchange)| >1 and P‐value <0.05 have been considered as DECs in TNBC. The workflow is briefly shown in Figure 1.
FIGURE 1.
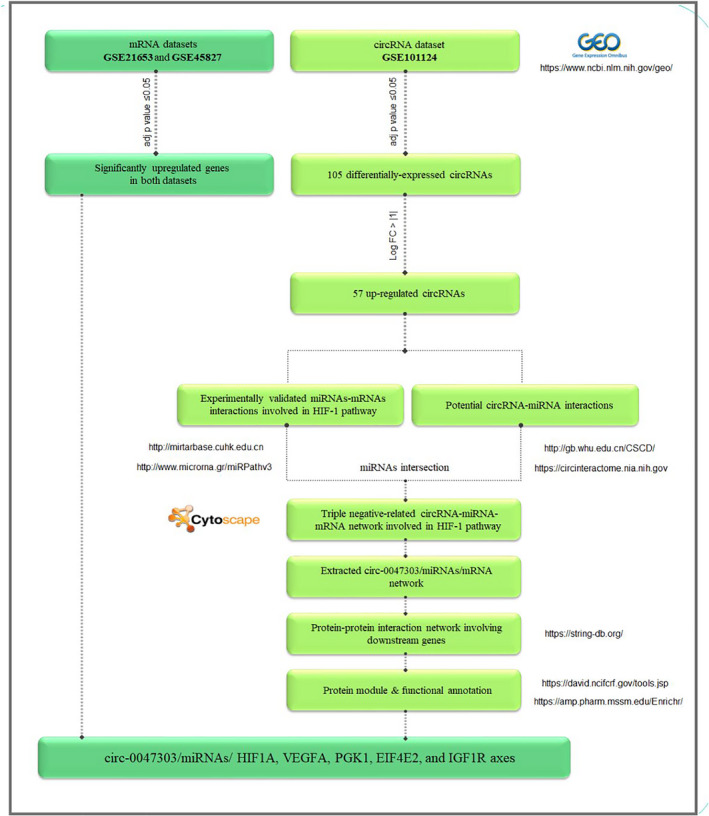
Workflow of in silico study
2.2. Investigation of differentially expressed genes (DEGs) in triple‐negative breast cancer (TNBC) versus normal mammary gland
Two microarray datasets of GSE21653 and GSE45827 containing protein‐coding genes in both TNBC and normal samples were selected. The former dataset comprised the data of 75 tumour and 29 normal samples and the latter 41 tumour and 11 normal samples. The results were used to select genes for further investigation.
2.3. Construction of circRNA/miRNA/mRNA regulatory network involved in HIF‐1 signalling pathway in TNBC
The circRNA/miRNA/mRNA regulatory network involved in the HIF‐1 signalling pathway was constructed according to the following steps:
(1) Prediction of potential interactions between the identified DECs and miRNAs using the CircInteractome database. 20 (2) The miRNAs involved in HIF‐signalling were identified using DIANA mirPath, 21 and consequently, their experimentally validated genes were achieved from DIANA TarBase. 22 Finally, the circRNA/miRNA/mRNA regulatory network was constructed by combining the data obtained in previous steps. Data visualization was performed using Cytoscape. 23
2.4. Construction of protein‐protein interaction (PPI) network and protein modules
STRING database 24 was used to establish the PPI network consisting of interactions among targets of miRNAs that exist in the constructed circRNA/miRNA/mRNA network. Next, the clustering algorithm ‘Molecular Complex Detection’ (MCODE) 25 was employed to delineate protein modules. Finally, enrichment analysis and functional annotation were performed using Enrichr and DAVID. 26
2.5. Construction of circ_0047303‐mediated ceRNA network involved in HIF‐1 signalling pathway in TNBC
In the next step, as circ_0047303 has the highest interaction with the miRNAs in the above‐mentioned network, the circ_0047303‐mediated ceRNA subnetwork was selected for further investigation. We hypothesized that some pivotal genes in HIF‐1 pathway could be upregulated through circ_0047303‐mediated miRNA suppression by circ_0047303‐miRNAs‐mRNAs axes. To test our hypothesis, we selected genes that met the following criteria: (a) Targeted by at least two miRNAs in the constructed circ_0047303‐mediated ceRNA subnetwork; (b) underwent an upregulation in TNBC according to microarray datasets GSE21653 and GSE45827; and (c) being involved in the identified protein modules. Thus, five genes, including HIF‐1A, VEGFA, PGK1, EIF4E2 and IGF1R, were selected for further validation. First, we evaluated the expression of these genes in TNBC tumours and adjacent normal tissues. Next, we examined the potential correlation between the expression of circ_0047303 and these genes in the samples.
2.6. Patients and samples collection
A total of 80 samples, including 40 primary TNBC and 40 matched normal tissues, were obtained from patients referred to Shahid Faghihi Hospital, Shiraz, Iran. None of the patients underwent radiotherapy or chemotherapy before sampling. All individuals have voluntarily participated in the study and have seen and signed the informed consent form. This study has been approved by the ethical committee of Fasa University of Medical Sciences under the ethical ID of IR.FUMS.REC.1398.125.
2.7. quantitative real‐time PCR
Extraction of total RNA was performed using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. RNA integrity and quantity were evaluated via gel electrophoresis and spectrophotometry (NanoDrop 2000, Thermo Scientific), respectively. For cDNAs synthesis from total RNA, the PrimeScript™ 1st Strand cDNA Synthesis Kit (TaKaRa Bio, Japan) was used. The sequence of circ_0047303 was obtained from the CircInteractome database, and specific primers were designed and used in real‐time PCR (Table S1). Quantitative real‐time PCR was carried out using RealQ Plus 2x Master Mix Green low Rox (Ampliqon) by Light‐Cycler96 Roche thermocycler. All reactions were performed in 10 µl and duplicated. Gene expression was quantitated using the 2−ΔΔCt method and normalized to the PUM1 gene as the internal control. PCR products were analysed by melting curve analysis and agarose gel electrophoresis. The circular junction of circ_0047303 was confirmed by Sanger sequencing.
2.8. Statistical analysis
Statistical analyses were performed using SPSS software (IBM). Student's t test was used to define statistically significant differences. One‐way analysis of variance (ANOVA) was adopted for nonparametric and parametric data. Rock‐curve analysis (ROC) was employed to investigate the diagnostic potential of circRNA biomarker(s). The Kaplan‐Meier method was used to investigate the association of circ_0047303 expression with disease‐free survival (DFS) of patients (data for 30 patients were available during a twenty‐month follow‐up).
3. RESULTS
3.1. DECs in TNBC versus normal mammary gland tissue based on microarray data
The GSE101124 dataset was retrieved from GEO and analysed to identify DECs. Comparison of circRNAs expression levels in TNBC versus normal mammary gland tissues revealed 105 DECs, including 57 up‐ and 48 down‐regulated circRNAs (Figure 2 and Table S2).
FIGURE 2.
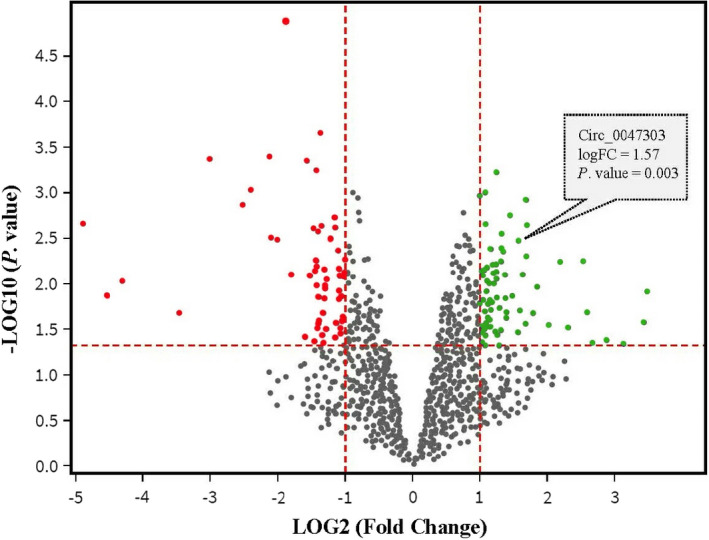
Volcano plot of differentially expressed circRNAs (DECs) in triple‐negative breast cancer (TNBC) compared with normal mammary tissues according to GSE101124. The cut‐off points of fold change (log2 scaled) and p value (log10 scaled) are shown by the red dash lines. The red points represent down‐expressed DECs in TNBC, and the green point represents up‐expressed DECs
3.2. DEGs in TNBC versus normal mammary gland tissue based on microarray data
The comparison of gene expression levels between TNBC and normal samples from GSE21653 and GSE45827 datasets reveals 2673 and 4123 DEGs, respectively (the data that support these findings are available from the corresponding author upon request).
3.3. The circRNA/miRNA/mRNA regulatory network involved in HIF‐1 signalling pathway in TNBC
Considering upregulated DECs, the potential circRNA‐miRNA sponging axes involved in HIF‐1 signalling were identified using CircInteractome and DIANA mirPath databases. Eleven miRNAs involved in HIF‐1 signalling pathway were identified that potentially have microRNA response elements (MREs) on sequences of upregulated DECs. Subsequently, 53 mRNAs were uncovered as the experimentally validated targets of the above‐mentioned miRNAs using DIANA TarBase. Finally, the TNBC‐related circRNA/miRNA/mRNA regulatory network involved in HIF‐1 signalling pathway was constructed (Figure 3). This network consists of 68 circRNA‐miRNA pairs and 93 miRNA‐mRNA pairs (Table S3).
FIGURE 3.
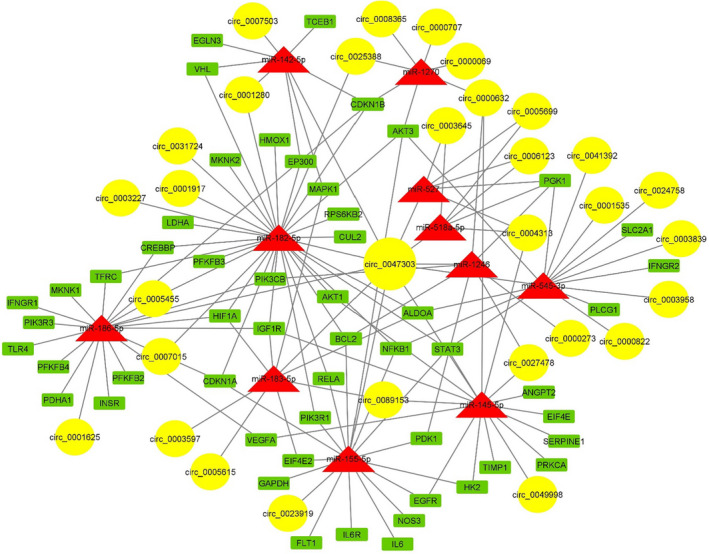
CircRNA/miRNA/mRNA regulatory network involving in the HIF‐1 signalling pathway in TNBC. Yellow circles, red triangles and green rectangles represent circRNAs, miRNAs and mRNAs, respectively. The node size of circRNAs corresponds to their out‐degree
3.4. The PPI network consisting of downstream targets of the circRNA/miRNA/mRNA regulatory network
Using the STRING database, the PPI network consisting of the downstream genes in the constructed circRNA/miRNA/mRNA network was visualized. This network consisted of 53 nodes and 421 edges (Figure S2A). Next, the protein modules were extracted from the network via MCODE based on densely connected regions of the PPI (Figure S2B). Upon enrichment analysis, it was revealed that these proteins positively regulate cell migration through angiogenesis and negative regulation of apoptosis (Table S4).
3.5. Validation of Circ_0047303 Up‐Expression In TNBC compared with tumour‐adjacent normal tissue
Results of analysing the microarray dataset GSE101124 demonstrated the significant up‐expression of circ_0047303 (hsa_circRNA_102333) in TNBC compared with normal mammary tissues. Here, using quantitative real‐time PCR, the expression of circ_0047303 was evaluated in 40 TNBC and 40 matched adjacent normal tissue samples. Results indicate that circ_0047303 is significantly upregulated in TNBC (Figure 4B and C). The junction site of circ_0047303 was confirmed by sanger sequencing (Figure 4A).
FIGURE 4.
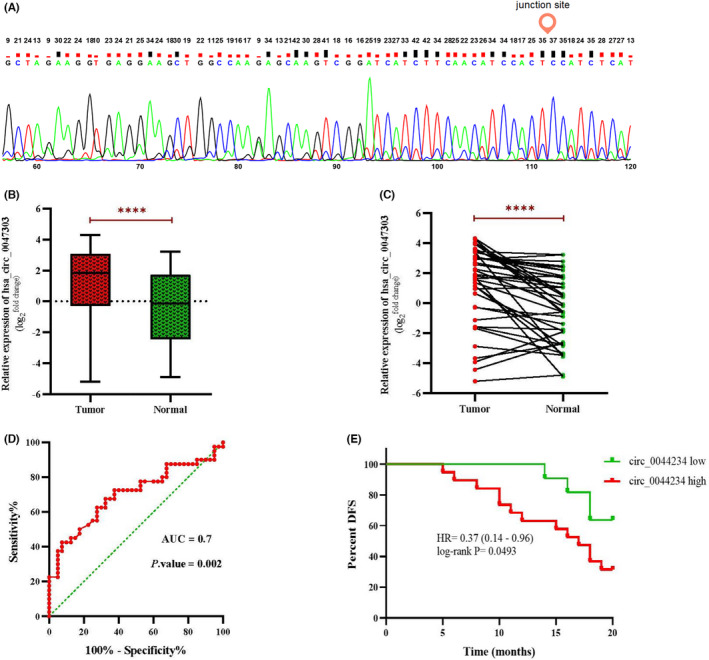
(A) Confirmation of the back‐splice junction site of circ_0047303 by Sanger sequencing. (B) and (C) The expression level of circ_0047303 detected by qRT‐PCR in TNBC samples and matched adjacent normal tissues. (D) Receiver operating characteristic (ROC) curve analysis of the expression of circ_0047303 in TNBC versus normal tissues; AUC, area under the ROC curve. (E) Association of circ_0047303 expression status with disease‐free survival in breast cancer. The red and green lines indicate patients with the high (N = 19) and low (N = 11) expression level of circ_0047303, respectively
3.6. The Circ_0047303 as a potential regulator of key genes in HIF‐1 signalling pathway
The constructed circRNA/miRNA/mRNA network indicates that circ_0047303, the up‐expressed circRNA with the highest degree, could potentially sponge/ inhibit multiple downstream miRNAs that function in the HIF‐1 signalling pathway. According to the criteria which have been formerly explained, the five genes HIF‐1A, VEGFA, PGK1, EIF4E2 and IGF1R were selected as downstream targets of circ_0047303‐mediated ceRNA network (Figure 5A). The expression analysis of the five genes by qRT‐PCR reveals that HIF‐1A, VEGFA and EIF4E2 underwent a significant upregulation in TNBC samples (Figure 5B). Moreover, regression analysis delineated a significant positive correlation between the relative expression of circ_0047303 and that of HIF‐1A, VEGFA and EIF4E2 genes (Figure 5C). Overall, most Pearson correlation coefficients between circ_0047303 and examined genes were positive (Figure 6A). The heat map from expression data of circ_0047303 and its five downstream genes shows a distinct expression pattern between TNBC and adjacent normal tissues, especially circ_0047303 and EIF4E2 (Figure 6B).
FIGURE 5.
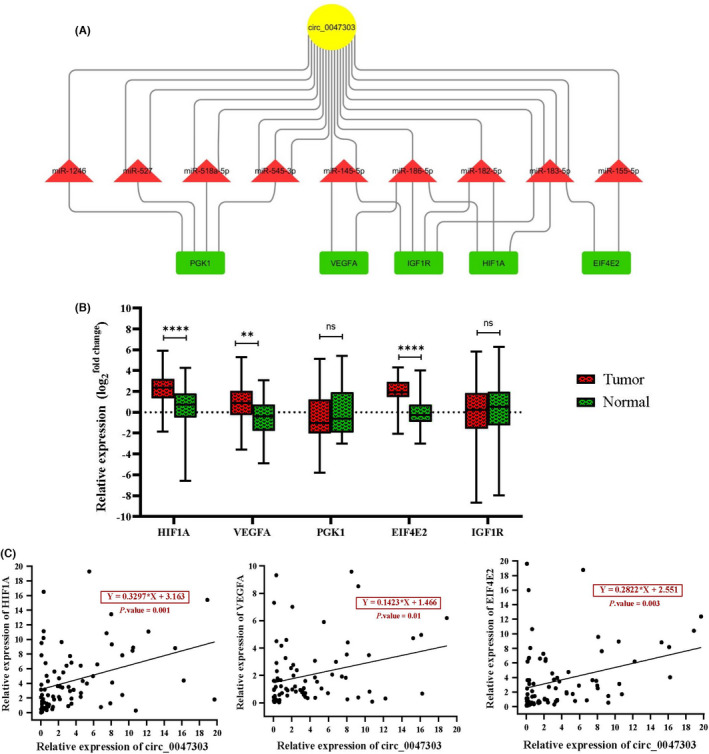
(A) Evaluated circ_0047303‐mediated ceRNA network involved in HIF‐1 signalling pathway in TNBC. The number of edges between circ_0047303 and miRNAs indicates the number of MRE on the circ_0047303 sequence. (B) Expression levels of HIF1A, VEGFA, PGK1, EIF4E2 and IGF1R was detected by qRT‐PCR in TNBC samples and matched adjacent normal tissues. (C) The expression correlation analysis of circ_0047303 with HIF1A, VEGFA and EIF4E2 expression levels in the studied samples. The regression equation has been calculated by linear regression analysis
FIGURE 6.
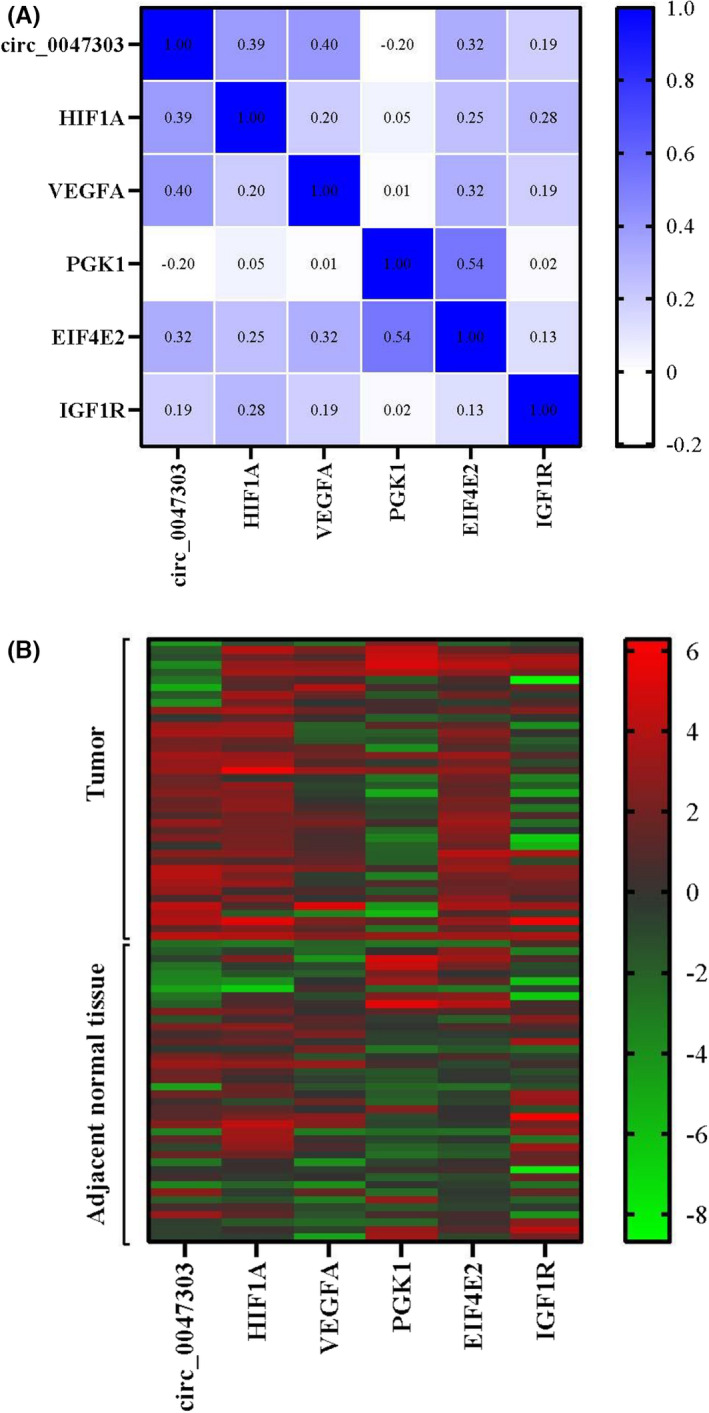
(A) Correlation matrix between circ_0047303 and its downstream gene targets has been represented by a colour scale based on Pearson's correlation coefficient. (B) Hierarchical clustering showing expression values (log2 transformed) of circ_0047303 and its downstream gene targets in TNBC samples and adjacent normal tissues. The expression values are arranged from red (high relative expression) to green (lower relative expression). Each row shows one sample, and each column demonstrates a transcript
3.7. Clinical implications of circ_0047303 as a diagnostic and prognostic biomarker
The ROC curve results show that circ_0047303 could serve as a potential diagnostic biomarker to discriminate TNBC from normal samples (AUC = 0.7) (Figure 4D). In other words, circ_0047303 can distinguish 70% of patients affected with TNBC with 75% specificity and sensitivity at the optimal cut‐off >1.085. The Kaplan‐Meier method indicates that patients with high circ_0047303 expression have a shorter DFS (Figure 4E). As far as correlation with clinicopathological characteristics of patients, circ_0047303 expression was significantly correlated with tumour size, tumour stage and lymph node metastasis. Therefore, we suggest a prognostic value for this circRNA: expression level of circ_0047303 was positively correlated with tumour size, tumour stage, and risk of metastasis (Table 1).
TABLE 1.
Association of circ_0047303 expression with clinicopathological features in BC patients
| Characteristic | Subgroups | No. of patients (%) | Median | p‐value |
|---|---|---|---|---|
| Age | <50 | 24 (60) | 5.89 | 0.852 |
| ≥50 | 16 (40) | 5.54 | ||
| Tumour size | <2 | 10 (25) | 1.59 | 0.021 |
| 2–5 | 26 (65) | 7.27 | ||
| >5 | 4 (10) | 6.23 | ||
| Histologic grade | G1 | 7 (17.5) | 7.54 | 0.523 |
| G2 | 20 (50) | 4.81 | ||
| G3 | 13 (32.5) | 6.22 | ||
| TNM stage a | I | 8 (21) | 1.01 | 0.0001 |
| II | 17 (44.7) | 3.55 | ||
| III | 11 (29) | 11.24 | ||
| IV | 2 (5.3) | 13.3 | ||
| Lymph nodes metastasis | Yes | 18 (45) | 8.02 | 0.003 |
| No | 22 (55) | 2.05 | ||
| Histologic type of invasive carcinoma | IDC | 35 (87.5) | 5.9 | 0.96 |
| ILC | 5 (12.5) | 4.69 |
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
The significant associations are shown in bold.
Two patients information missing.
4. DISCUSSION
For the first time, this study has introduced the involvement of circ_0047303/miRNA/mRNA ceRNA network in triple‐negative breast cancer via HIF‐1 signalling. Our results demonstrated remarkably enhanced levels of circ_0047303 and three angiogenesis‐associated genes including HIF‐1, EIF4E2 and VEGFA in triple‐negative samples. Furthermore, our data suggest that circ_0047303 could serve as a potential diagnostic (AUC = 0.7) and prognostic biomarker for TNBC. Our study was on the most aggressive BC subtype, which is associated with a poor outcome and the highest metastasis rate compared to other subtypes, 27 , 28 and leads to rapid deterioration and distant metastasis in patients. 29
We looked up potential DE circRNAs that underly TNBC progression through their function in HIF‐1 signalling pathway. These RNAs harbour MREs that bind to and sponge specific miRNAs and regulate the expression of their target genes. 30 , 31
Results of microarray data analysis showed that circ_0047303 was significantly upregulated in TNBC compared to normal mammary tissues. Moreover, we found circ_0047303 shows the highest degree in the constructed circRNA/miRNA/mRNA network (Figure 3). According to the selection criteria adopted in this study, the downstream genes of circ_0047303‐mediated ceRNA network should be: (i) targeted by at least two miRNAs predicted to be sponged by circ_0047303; (ii) upregulated in TNBC according to microarray data analysis results; and (iii) involved in the identified protein modules. Five HIF‐1 signalling‐related genes, including HIF‐1A, VEGFA, PGK1, EIF4E2 and IGF1R, were determined as downstream targets of circ_0047303‐mediated ceRNA axes (Figure 5A). We assume that these genes could be involved in the circ_0047303/miRNA/mRNA network and that in the case of circ_0047303 upregulation, miRNAs are sponged away from target mRNAs and can no longer inhibit their expression. Thus, it might explain the upregulation of their target genes.
Our experimental results indicate that circ_0047303, also known as hsa_circRNA_102333, is significantly upregulated in TNBC compared with adjacent normal tissues, further confirming the findings of microarray data analysis. Moreover, we found that the expression signature of circ_0047303 has a diagnostic value for TNBC with AUC 0.7 at the optimal cut‐off >1.085 (75% specificity and sensitivity). We also examined the prognostic value of circ_0044234 expression in terms of DFS in TNBC patients. The data show that the high expression level of circ_0047303 in the tumour samples tends to lower DFS (p = 0.049). As the follow‐up time in the current study is short (20 months), it is suggested that the prognostic value of circ_0047303 be investigated in a larger sample size and a longer follow‐up time in future researches.
Additionally, association analysis of the patients’ clinicopathological features and the expression level of circ_0047303 revealed a significant positive correlation between the expression of circ_0047303 and tumour size, lymph node metastasis, and TNM stage. Higher TNM stage and larger tumour size were correlated with higher expression of circ_0047303. Besides, tumours with lymph node metastasis had a higher expression rate compared to non‐metastatic tumours. In conclusion, we suggest that circ_0047303 could serve as a novel potential diagnostic and prognostic biomarker in TNBC. The circ_0047303 is an exonic circRNA derived from exon 3 of ZNF521 gene located at chromosome 18. Upregulation of circ_0047303 has also been reported in hepatocellular carcinoma. 32 However, the current knowledge of the oncogenic role of this circRNA in different malignancies is relatively limited.
In the following step, the expression of five downstream genes in circ_0047303‐mediated ceRNA axes (Figure 5a) was evaluated by qRT‐PCR. The results show a significant upregulation of HIF‐1A, VEGFA and EIF4E2 genes in TNBC (Figure 5b).
It has been shown that HIF‐1 is involved in tumorigenesis and/or tumour progression: overexpression of HIF‐1α or HIF‐2α was linked with mortality in several cancers, including BC. 33 Currently, inhibition of HIF‐1 is considered as a cancer therapy approach, and many drugs have been developed that serve this purpose. 33 Given that HIF‐1 is a crucial transcription factor to VEGFA expression, 34 the drug‐mediated lack of HIF‐1 might also explain the mechanism underlying decreased VEGFA expression. High plasma levels of VEGFA, an important angiogenic factor, had a positive correlation with advanced tumour stages in BC patients. 35 Similarly, Roberti, et al. demonstrated that VEGFA protein expression is a potential prognostic biomarker for TNBC. 36 It has been recently shown that VEGF signalling exerts immune suppressive effects in BC tumours. 37 In hypoxia, the eukaryotic initiation factor 4E type 2 (EIF4E2) embarks on the translation of HIF‐2α‐bound mRNAs that contain hypoxia response elements. 38 Silencing of EIF4E2 in MDA‐MB‐231 triple‐negative cell line indicated a remarkable decrease in tumour cell migration and invasion during hypoxia. 38
Our data suggest a significant positive correlation between the expression of circ_0047303 and that of HIF‐1A, VEGFA and EIF4E2 genes (Figure 5C). Interestingly, circ_0047303 harbours four MREs for the seed region of miR‐183‐5p (Figure 5A), which could silence both HIF‐1A and EIF4E2 genes. Therefore, we suggest that circ_0047303 might modulate the expression of these genes in hypoxia, and the upregulation of this circRNA results in the up‐expression of HIF‐1A and EIF4E2 in TNBC through decreasing the intracellular levels of miR‐183‐5p.
There are contradictory data regarding the dysregulation and function of miR‐183 in BC. In line with our hypothesis, some studies have documented its tumour‐suppressive role. For instance, upregulation of miR‐183 prevents migration and invasion of breast tumours. 39 Recently, Anderson, et al. reported significant down‐expression of miR‐183‐5p in post‐EMT (epithelial‐mesenchymal transition) breast cancer cells. Moreover, restoring the expression of miR‐183‐5p with an exogenous mimic resulted in a significant decrease in BC cell invasion and migration. 40 In contrast, several studies revealed the upregulation and oncogenic role of miR‐183‐5p in BC. 41 , 42 These inconsistent observations could be caused by different expression profiles between various intrinsic BC subtypes. Here, for the first time, we propose the potential role of circ_0047303/ mir‐183‐5p/ HIF‐1A and EIF4E2 regulatory axes in TNBC pathogenesis according to our experimental and in silico investigation.
Another target of circ_0047303 is miR‐145 for which it harbours two MREs (Figure 5A). Regarding direct suppression of VEGFA by miR‐145, 43 we suggest that up‐expressed circ_0047303 could modulate the expression of VEGFA through sponging miR‐145 in TNBC. Various studies have shown the downregulation of miR‐145 in BC 44 and TNBC tissues and cell lines. 45 , 46 There is evidence that miR‐145 upregulation in BC exerts a tumour repressive effect through inhibition of angiogenesis and cell invasion. 43 Therefore, miR‐145 has been previously suggested to act as a tumour suppressor, and in the current study, we have uncovered a potential underlying mechanism to suppress miR‐145‐5p in TNBC: up‐expression of circ_0047303 as a sponge! Altogether, we propose the TNBC‐related ceRNA axes comprising circ_0047303/miR‐183‐5p/HIF‐1 and EIF4E2 as well as circ_0047303/miR‐145‐5p/VEGFA.
Although in silico findings in this study were experimentally validated, a certain shortcoming here is the lack of in vivo/in vitro approaches such as animal modelling and cell culturing, respectively. Such studies provide functional evaluation of circ_0047303 and further facilitate understanding the potential role of circ_0047303 and molecular interactions within the circRNA/miRNA/mRNA network in angiogenesis through HIF‐signalling pathway.
In conclusion, we used an integrative systems biology‐directed strategy to study the molecular mechanism underlying angiogenesis in TNBC. By combining microarray datasets and using bioinformatics tools, we constructed a circRNA/miRNA/mRNA network involved in HIF‐1 signalling pathway in TNBC. We found circ_0047303 as the highest degree centrality node in this ceRNA network, which showed a significant up‐expression in triple‐negative tumours. Our data indicate that circ_0047303 has a potential value as a diagnostic and prognostic biomarker in TNBC. In the next step, we delineated the novel circ_0047303‐mediated ceRNA axes involved in the aggressive behaviour of TNBC using an in silico‐in vitro approach. Our data suggest that upregulation of three angiogenesis‐associated genes including HIF‐1, EIF4E2 and VEGFA in TNBC could be mediated by circ_0047303 through sponging the tumour‐suppressive miRNAs. This study, for the first time, shines a light on the role of circRNAs in HIF‐1 signalling pathway in TNBC and suggests that circ_0047303 is a promising molecular biomarker and/or therapeutic target. Nevertheless, future studies are required to examine the possible clinical applications.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTIONS
F.D. and Y.M.: Conceptualization. F.D.: Methodology. F.D. and A.D.: Validation. F.D. and M.B.: Formal analysis. F.D., Z.N. and Z.F.: Investigation. Y.M and B.M.: Resources. F.D. and M.M.: Writing‐original draft preparation. F.D., Y.M. and Z.N.S.F.: Writing‐review and editing. F.D., M.M. and E.V.: Visualization. Y.M.: Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Supporting information
Fig S1‐S2
Table S1‐S4
ACKNOWLEDGMENTS
This work was supported by the department of medical genetics, Fasa University of Medical Sciences (FUMS), Fasa, Iran (grant number 97397).
Darbeheshti F, Mahdiannasser M, Noroozi Z, et al. Circular RNA‐associated ceRNA network involved in HIF‐1 signalling in triple‐negative breast cancer: circ_0047303 as a potential key regulator. J Cell Mol Med. 2021;25:11322–11332. doi: 10.1111/jcmm.17066
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article. Moreover, the analysed data of two GEO datasets GSE21653 and GSE45827 are available from the corresponding author upon request.
REFERENCES
- 1. Wang Z, Jiang Q, Dong C. Metabolic reprogramming in triple‐negative breast cancer. Cancer Biol Med. 2020;17:44‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple‐negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jalalvand M, Darbeheshti F, Rezaei N. Immune checkpoint inhibitors: review of the existing evidence and challenges in breast cancer. Immunotherapy. 2021;13:587‐603. [DOI] [PubMed] [Google Scholar]
- 4. Mansoori Y, Zendehbad Z, Askari A, et al. Breast cancer‐linked lncRNA u‐Eleanor is upregulated in breast of healthy women with lack or short duration of breastfeeding. J Cell Biochem. 2019;120:9869‐9876. [DOI] [PubMed] [Google Scholar]
- 5. Chang JC, Hilsenbeck SG, Fuqua SAW. Genomic approaches in the management and treatment of breast cancer. Br J Cancer. 2005;92:618‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bharti SK, Mironchik Y, Wildes F, et al. Metabolic consequences of HIF silencing in a triple negative human breast cancer xenograft. Oncotarget. 2018;9:15326‐15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi R, Liao C, Zhang Q. Hypoxia‐driven effects in cancer: characterization, mechanisms, and therapeutic implications. Cells. 2021;10:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong C C‐L, Gilkes DM, Zhang H, et al. Hypoxia‐inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci U S A. 2011;108:16369‐16374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Generali D, Berruti A, Brizzi MP, et al. Hypoxia‐inducible factor‐1α expression predicts a poor response to primary chemoendocrine therapy and disease‐free survival in primary human breast cancer. Clin Cancer Res. 2006;12:4562‐4568. [DOI] [PubMed] [Google Scholar]
- 11. Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia‐inducible factor 1 (HIF‐1) transcriptional activity on targets like vascular endothelial growth factor (VEGF)*. J Biol Chem. 2014;289:3352‐3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darbeheshti F, Rezaei N, Amoli MM, Mansoori Y, Tavakkoly BJ. Integrative analyses of triple negative dysregulated transcripts compared with non‐triple negative tumors and their functional and molecular interactions. J Cell Physiol. 2019;234:22386‐22399. [DOI] [PubMed] [Google Scholar]
- 13. Klinge CM. Non‐Coding RNAs in breast cancer: intracellular and intercellular communication. Non‐Coding RNA. 2018;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mansoori H, Darbeheshti F, Daraei A, et al. Expression signature of lncRNA APTR in clinicopathology of breast cancer: its potential oncogenic function in dysregulation of ErbB signaling pathway. Gene Reports. 2021;23:101116. [Google Scholar]
- 15. Zeng K, He B, Yang BB, et al. The pro‐metastasis effect of circANKS1B in breast cancer. Mol Cancer. 2018;17:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang S‐J, Wang D‐D, Zhou S‐Y, et al. Identification of circRNA‐miRNA networks for exploring an underlying prognosis strategy for breast cancer. Epigenomics. 2020;12:101‐125. [DOI] [PubMed] [Google Scholar]
- 17. Abdollahzadeh R, Daraei A, Mansoori Y, Sepahvand M, Amoli MM, Tavakkoly‐Bazzaz J. Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: a new look at hallmarks of breast cancer. J Cell Physiol. 2019;234:10080‐10100. [DOI] [PubMed] [Google Scholar]
- 18. Sang M, Wu M, Meng L, et al. Identification of epithelial‐mesenchymal transition‐related circRNA‐miRNA‐mRNA ceRNA regulatory network in breast cancer. Pathol Res Pract. 2020;216:153088. [DOI] [PubMed] [Google Scholar]
- 19. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, Gorospe M. CircInteractome: a web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 2016;13:34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vlachos IS, Zagganas K, Paraskevopoulou MD, et al. DIANA‐miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43:W460‐W466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vlachos IS, Paraskevopoulou MD, Karagkouni D, et al. DIANA‐TarBase v7.0: indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2015;43:D153‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498‐2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2014;43:D447‐D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90‐W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mathe A, Wong‐Brown M, Morten B, et al. Novel genes associated with lymph node metastasis in triple negative breast cancer. Sci Rep. 2015;5:15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Darbeheshti F, Izadi P, Emami Razavi AN, Kamali F, Yekaninejad MS, Tavakkoly BJ. Significance of EGFR mRNA expression in luminal and triple negative breast tumors. Int J Cancer Manag. 2018;11:e9763. [Google Scholar]
- 29. Rakha EA, Chan S. Metastatic triple‐negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23:587‐600. [DOI] [PubMed] [Google Scholar]
- 30. Bai S, Wu YY, Yan Y, et al. Construct a circRNA/miRNA/mRNA regulatory network to explore potential pathogenesis and therapy options of clear cell renal cell carcinoma. Sci Rep. 2020;10:13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kadkhoda S, Darbeheshti F, Rezaei N, et al. Investigation of circRNA‐miRNA‐mRNA network in colorectal cancer using an integrative bioinformatics approach. Gastroenterol Hepatol Bed Bench. 2021;14:141‐153. [PMC free article] [PubMed] [Google Scholar]
- 32. Ou M, Lin H, Gong W, et al. Comprehensive analysis of circRNA expression patterns in small hepatocellular carcinoma by integrating circRNA and gene expression data. Int J Clin Exp Med. 2017;10:2858‐2865. [Google Scholar]
- 33. Semenza GL. Defining the role of hypoxia‐inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pagès G, Pouysségur J. Transcriptional regulation of the vascular endothelial growth factor gene–a concert of activating factors. Cardiovasc Res. 2005;65:564‐573. [DOI] [PubMed] [Google Scholar]
- 35. Ławicki S, Zajkowska M, Głażewska EK, Będkowska GE, Szmitkowski M. Plasma levels and diagnostic utility of VEGF, MMP‐9, and TIMP‐1 in the diagnosis of patients with breast cancer. Onco Targets Ther. 2016;9:911‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberti MP, Arriaga JM, Bianchini M, et al. Protein expression changes during human triple negative breast cancer cell line progression to lymph node metastasis in a xenografted model in nude mice. Cancer Biol Ther. 2012;13:1123‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cierna Z, Smolkova B, Cholujova D, et al. Decreased levels of circulating cytokines VEGF, TNF‐β and IL‐15 indicate PD‐L1 overexpression in tumours of primary breast cancer patients. Sci Rep. 2021;11:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelly NJ, Varga JFA, Specker EJ, Romeo CM, Coomber BL, Uniacke J. Hypoxia activates cadherin‐22 synthesis via eIF4E2 to drive cancer cell migration, invasion and adhesion. Oncogene. 2018;37:651‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lowery AJ, Miller N, Dwyer RM, Kerin MJ. Dysregulated miR‐183 inhibits migration in breast cancer cells. BMC Cancer. 2010;10:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anderson O, Guttilla Reed IK. Regulation of cell growth and migration by miR‐96 and miR‐183 in a breast cancer model of epithelial‐mesenchymal transition. PLoS One. 2020;15:e0233187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Macedo T, Silva‐Oliveira RJ, Silva VAO, Vidal DO, Evangelista AF, Marques MMC. Overexpression of mir‐183 and mir‐494 promotes proliferation and migration in human breast cancer cell lines. Oncol Lett. 2017;14:1054‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng Y, Xiang G, Meng Y, Dong R. MiRNA‐183‐5p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting the PDCD4. Reprod Biol. 2016;16:225‐233. [DOI] [PubMed] [Google Scholar]
- 43. Zou C, Xu Q, Mao F, et al. MiR‐145 inhibits tumor angiogenesis and growth by N‐RAS and VEGF. Cell Cycle. 2012;11:2137‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iorio MV, Ferracin M, Liu C‐G, et al. MicroRNA gene expression deregulation in human breast cancer. Can Res. 2005;65:7065. [DOI] [PubMed] [Google Scholar]
- 45. Radojicic J, Zaravinos A, Vrekoussis T, Kafousi M, Spandidos DA, Stathopoulos EN. MicroRNA expression analysis in triple‐negative (ER, PR and Her2/neu) breast cancer. Cell Cycle. 2011;10:507‐517. [DOI] [PubMed] [Google Scholar]
- 46. Tang L, Wei D, Yan F. MicroRNA‐145 functions as a tumor suppressor by targeting matrix metalloproteinase 11 and Rab GTPase family 27a in triple‐negative breast cancer. Cancer Gene Ther. 2016;23:258‐265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Table S1‐S4
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article. Moreover, the analysed data of two GEO datasets GSE21653 and GSE45827 are available from the corresponding author upon request.


