Table 1. Optimization of Reaction Conditions for the Synthesis of (S)-1a.
| entry | solvent | yield (S)-1 (%)b | aniline (%)b |
|---|---|---|---|
| 1 | THF | 23 | 77 |
| 2 | EtOH | 21 | 59 |
| 3 | toluene | 52 | 21 |
| 4 | acetone | 51 | 9 |
| 5 | CH2Cl2 | 47 | 4 |
| 6 | CHCl3 | 57 | 6 |
| 7 | MeCN | 47 | 6 |
| 8 | EtOAc | 65 | 5 |
| 9 | PhCF3 | 66 | 4 |
| 10 | DMF | –b | – |
| 11 | H2O | 0b | 1 |
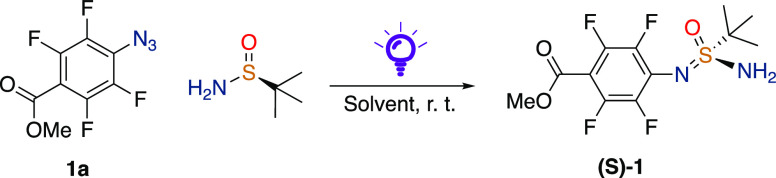
Reaction conditions: azide (0.075 mmol, 0.05 M), (S)-tert-butylsulfinamide (0.15 mmol, 1.5 equiv), degassed solvent (1.5 mL), and 390 nm Kessil LED light, 1.5 h.
Determined by 1H NMR with an internal standard.