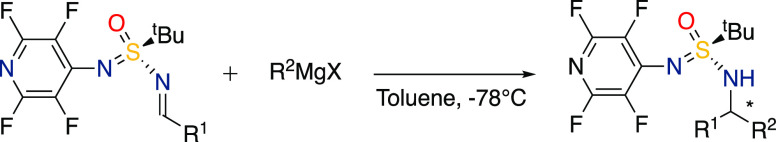
Table 4. Scope of the Addition of Grignard Reagents to Imines Derived from SIAsa.
| entry | R1 | R2 | X | yieldb | drc |
|---|---|---|---|---|---|
| 1 | tBu | Ph | Br | 86 | 95:5 |
| 2 | tBu | 3-methoxy-C6H4 | Br | 90 | 96:4 |
| 3 | tBu | 4-chloro-C6H4 | Br | 98 | 93:7 |
| 4 | tBu | Me | Br | 86 | 67:33 |
| 5 | tBu | iPr | Cl | ||
| 6 | Ph | 3-methoxy-C6H4 | Br | 85 | 92:8 |
| 7 | Ph | 4-chloro-C6H4 | Br | 85 | 92:8 |
| 8 | Ph | Me | Br | 80 | 84:16d |
| 9 | 4-chloro-C6H4 | Ph | Br | 86 | 84:16 |
| 10 | 4-chloro-C6H4 | 3-methoxy-C6H4 | Br | 90 | 94:6 |
Reaction conditions: imine (0.05 mmol, 1 equiv), Grignard reagent (2.5 equiv), toluene (0.5 mL), −78 to r.t.
The yield was determined by 1H NMR spectroscopy using tert-butyl methyl ether as the internal standard.
Determined by 1H NMR spectroscopy or chiral HPLC.
Reaction performed in CH2Cl2.