Abstract
This manuscript describes our studies of the class of natural products known as the rubellins, culminating in the total synthesis of (+)-rubellin C. These anthraquinone-based natural products contain a variety of stereochemical and architectural motifs, including a 6–5-6-fused ring system, 5 stereogenic centers, and a central quaternary center. Herein, we report our development of a strategy to target the stereochemically dense core and anthraquinone nucleus, including approaches such as a bifunctional allylboron and vinyl triflate reagent, an anthraquinone benzylic metalation strategy, and a late-stage anthraquinone introduction strategy. Our studies culminate in a successful route to highly functionalized anthraquinone-based natural product scaffolds and a stereoselective total synthesis of (+)-rubellin C. These strategies and outcomes will aid in synthetic planning toward anthraquinone-based natural products of high interest.
Graphical Abstract

INTRODUCTION
Rubellins A and B (1 and 2) were initially isolated as heterodimeric anthraquinone metabolites from the phytopathogenic fungus Mycosphaerella rubella in 1986 in Italy, causing pigmentation and damage to a rare medicinal plant Angelica sylvestris, with rubellins C and D (3 and 4) following soon after (Figure 1).1,2 Research surrounding the compounds lay dormant for years until the isolation of the rubellins from another more common fungus in Germany, Ramularia collo-cygni, the emerging pathogen responsible for barley leaf disease in Europe.3 Further investigation of R. collo-cygni yielded natural products with similar core structures, including rubellins E and F (5 and 6), uredinorubellins A and B (9 and 10), caeruleoramularin (11), and 14-dehydro rubellin D (12), all direct rubellin family members.4
Figure 1.
Rubellin family and related natural products.
A biosynthesis from R. collo-cygni was carried out by the Liebermann group in 2006.4 Small quantities of various rubellins could be isolated from the fungi, and 13C labeling via [U-13C6]-glucose allowed for confirmation of an anthraquinone dimerization through isolation of the anthraquinone monomers chrysophanol (7) and helminthosporin (8) (Scheme 1). An enzyme-mediated dimerization was postulated to give a bisanthraquinone species (17), which undergoes enzymatic reduction and oxidation events to provide the rubellin natural products.4,5 Related natural products isolated from different fungi include the torrubiellins (13 and 14), melrubiellins (15 and 16), and solanrubiellins, indicating that many fungal species may have the enzymes responsible for such chemistries.6–8
Scheme 1.
Biosynthetic Hypothesis of Rubellin Natural Products
Reported Biological Activities.
In the initial studies of rubellins A and B (1 and 2), no biological activities were reported.1 However, subsequent studies showed a light-dependent antibiotic ability of the rubellins.2 Photodynamic properties were observed, with all of the rubellins possessing the ability to act as lipoperoxidative agents on select substrates, which is supplemented by the presence of these natural products in phytotoxic fungi.9
Over a decade later, further investigation into the photodynamic ability of the rubellins confirmed their propensity to undergo light-mediated reactions.4,10 Mechanistically, this process is thought to occur in a mixture of photodynamic type I and II reactions, where the rubellin core accesses an excited state via the energy of a photon. This energy is transmitted to oxygen to produce a reactive oxygen species in the form of singlet oxygen or the superoxide radical via electron donation, which then decomposes to the hydroxyl radical through hydrogen peroxide (Scheme 2). The reactive oxygen species can then oxidize fatty acids in the cell wall. This mechanism of action is particularly damaging to plant cell walls and is similar to that of the related natural product cercosporin, synthesized for the first time in 2010.11
Scheme 2.
Light-Activation Hypothesis of Rubellin Natural Products
A full biological profile was conducted in 2009 on rubellins B (2), C (3), D (4), E (5), and caeruleoramularin (11).12 Low micromolar light-dependent antibiotic activities were observed against Gram-positive but not Gram-negative bacteria. Cytotoxicity was observed in a variety of cell lines with some light dependency observed as well; however, no significant selectivity between cell types was seen. Intriguingly and similar to other anthraquinone-containing natural products, the rubellins were found to potently inhibit and deaggregate the formation of paired-helical filaments of tau protein, a therapeutically relevant biomarker in Alzheimer’s disease, implicating this scaffold as the basis for tools or therapeutics for the neurodegenerative tauopathies.13 Our further studies on this family of natural products were based partially on the reported ability of the rubellins to deaggregate neurotoxic tau aggregates.
RESULTS AND DISCUSSION
Retrosynthetic Analysis.
None of the discussed rubellin natural products (1–16) have been conquered through total synthesis since their initial discovery (1 and 2) over 30 years ago, an odd fact due to the recent syntheses of other multilinked anthraquinone natural products. The rubellin core is beset with several synthetic challenges, including the quaternary center buried in an array of five contiguous stereocenters, the central 6–5-6-fused ring system, and the assortment of oxidation levels (Figure 2). We were particularly interested in developing a synthetic approach to rubellin C (3) because of the exceptional challenge of accessing a Csp3–Csp3 hybridized “attached ring system”, where the quaternary C16 carbon in a ring is joined through a single carbon–carbon bond to a stereochemically defined lactone.14 Given the prevalence of the congested attached ring motif in other natural products, we envisioned that a selective approach to this core structure would have broad utility in complex molecule synthesis.15–17 We describe in detail our synthetic studies and our reported total synthesis of (+)-rubellin C.18
Figure 2.
Rubellin architecture synthetic challenges.
Our retrosynthesis of (+)-rubellin C (3) revolved around two major disconnections (Scheme 3). Our first disconnection is of the Csp2–Csp3 C5–C11 bond via a diastereoselective intramolecular Heck reaction from phenol 18 as its corresponding triflate, where the stereochemistry of the carbopalladation is directed by the quaternary center.19 We reasoned that the key hindered ring fusion present in anthraquinone 18 could be selectively accessed through an allylation in an ordered transition state 19. Properly designed synthon allyl anion 21 combined with phthalaldehyde 20 would give access to such a transformation. A stereochemically defined allylic nucleophile corresponding to the allyl anion synthon 21 would have to engage the electrophilic aldehyde 20 through the correct carbon (α- vs γ-addition), on the correct face, and with the correct orientation of the electrophile, distinguishing between eight possible isomers of the coupled product containing the two core stereocenters; the resulting homoallylic alcohol would then close on the ortho-ester of the aldehyde partner to form the attached ring system. We proposed a sterically hindered allylboron allylation that would translate stereochemistry through a closed transition state 19.20,21 We then set out to produce the stereochemically enriched allylating reagent 21 from a chiral pool starting material (22) and define the ring system that would be necessary for the allylating reagent to incorporate the anthraquinone functionality.
Scheme 3.
Retrosynthetic Analysis of (+)-Rubellin C
Many possible routes exist to gain access to the required intermediates to assemble the rubellin system through our proposed key allylative and Heck chemistries (Scheme 4). We attempted three different strategies to access necessary intermediates, which will be discussed as the first-, second-, and third-generation synthetic approaches. Our strategies involved an initial investigation of a bifunctional reagent equipped with an allylboronic ester and vinyl triflate (23), the production of an electrophile (24) and anthraquinone organometallic reagent (25), and a late-stage introduction of the anthraquinone through a Hauser annulation.
Scheme 4.
First-, Second-, and Third-Generation Retrosynthetic Approaches
First-Generation Synthetic Approach.
Our first-generation synthetic approach relied on the construction of the allylative precursor through a bifunctional reagent equipped with both allylboron and vinyl triflate moieties (23). This could undergo an initial transition-metal-mediated coupling to install the benzylic anthraquinone species followed by allylative chemistry to form the necessary Csp3–Csp3-attached ring system. The bifunctional reagent could be derived from enone 28, a product of a diastereoselective 1,4-addition of boron into enone 29, which could be generated from D-(−)-quinic acid (22) (Scheme 5).
Scheme 5.
Retrosynthesis of Bifunctional Lynchpin 23
To access the necessary enantioenriched enone 29 from D-(−)-quinic acid (22), many methods from the literature were attempted (Scheme 6). Ultimately, a piecemeal approach was taken, as many of the multistep procedures were not reproducible in our hands.22–24 Starting from D-(−)-quinic acid (22), an initial lactonization and acetonide protection afforded lactone 30 on decagram scale (maximum scale performed 100 g).21 Lithium aluminum hydride reduction gave access to triol 31, which became a bottleneck in scale for the synthesis due to concentration requirements. Triol 31 was oxidatively cleaved to hydroxyketone 32 with Shing’s reagent (NaIO4 adsorbed on silica gel).22,23 These steps could be run on decagram scale with no chromatographic purification required, and hydroxyketone 32 served as a vital branch point intermediate in early-stage synthesis. Hydroxyketone 32 was reduced to diol 33 as a mixture of diastereomers and subjected to pyridine and p-toluenesulfonyl chloride to produce a mixture of diastereomeric and constitutional isomers of the monotosylated quinic acid derivative (undesired 34, desired 35).22 Swern oxidation with excess base facilitated elimination to the desired enone 29.22
Scheme 6.
Forward Synthesis of Enone 29
We then examined the 1,4-borylation and triflation reactions on the enone substrate 29 (Scheme 7). A copper-catalyzed borylation reported by Miyaura utilizing copper(I) chloride, lithium chloride, bis(pinacolato)diboron, and potassium acetate in N,N-dimethylformamide (DMF) furnished low yields of one diastereomer of the 1,4-borylated enone 28.25 NOE experiments were unsuccessful in determining the relative stereochemistry of the new carbon–boron bond; however, we surmised that the convex face of the system was much more accessible. An increase in yield was observed upon switching to a copper/iron system as reported by Ramón and Yus, where iron oxide is doped with copper to yield a magnetic catalyst.26 A similar mechanism is thought to control both reactions: the inorganic base forms methoxide, which chelates to the empty p-orbital of one boron unit of bis(pinacolato)diboron. This facilitates transmetallation of boron to copper to form a nucleophilic copper–boron bond, which then undergoes 1,4-addition to enone 29 in a diastereoselective manner on presumably the opposite face of the acetonide. This reaction, although early in our studies, proved vital to our understanding of the stereochemical control we would encounter throughout the rest of our synthetic strategies.
Scheme 7.
Diastereoselective 1,4-Borylations of Enone 29
Triflation of the resulting borylated compound 28 garnered the undesired regioisomer 36 as the major species in all conditions (Table 1). Although the carbon–boron bond survived the basic conditions, deprotonation occurred at C15 rather than at C11. Examination of various bases, including strong bases such as lithium diisopropylamide (LDA) and weaker bases such as 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU), in various solvents and temperatures led to the undesired isomer with a variety of triflating agents. This reactivity could be rationalized through the electronegativity differences of C15 and C11 as well as the steric hindrance around pinacol at nearby C12. Furthermore, the hydrogen at the undesired C15 is also likely oriented antiperiplanar to the acetonide oxygen at C14 due to negative hyperconjugation, which would further stabilize the resulting anion. This makes the undesired regioisomer 36 both the kinetically and thermodynamically favored products, which forced us to rethink our synthetic approach.
Table 1.
Regioselective Triflation of Enone 28a

|
Comins’ reagent: N-(5-chloropyridin-2-yl)-N-(methanesulfonyl)methanesulfonamide.
Second-Generation Synthetic Approach.
Our second-generation approach replaced the transition-metal-coupling step with a conjugate addition of a benzylic anthraquinone organometallic species (Scheme 8). In both synthetic routes, we required an organometallic benzylic anthraquinone; in this case, instead of coupling to an inaccessible vinyl triflate 23, we could attempt addition of the nucleophilic benzylic reagent to the opposite constitutional isomer of the previous enone 29, namely, enone 24, which was accessed through hydroxyketone 32 via mesylation and elimination.23 This gave access to the necessary Michael acceptor 24 for the desired nucleophilic benzylic anthraquinone 25.
Scheme 8.
Retrosynthesis of Putative Organometallic Species 25
Although no direct benzylic anthraquinone organometallic exists in the literature, anthraquinones have been shown to resist additions of some organometallic reagents, such as Grignard reagents, and can be successfully metallated at their aromatic positions (Scheme 9).27–29 Major questions revolved around the metal insertion event. Would a low-valent metal form the organometallic reagent at the benzylic position or reduce the anthraquinone to its anthracene derivative? Furthermore, would the resulting reagent be nucleophilic enough to add into the carbonyls of the anthraquinone, even though they are sterically hindered and electronically deactivated in this particular system? However uncertain, we attempted to synthesize multi-substituted anthraquinone 25 (or the anthracene equivalent) that could be possibly metallated at the benzylic position.
Scheme 9.
Examples of Poor Nucleophilic Reactivity toward the Anthraquinone C9/C10 Carbonyls
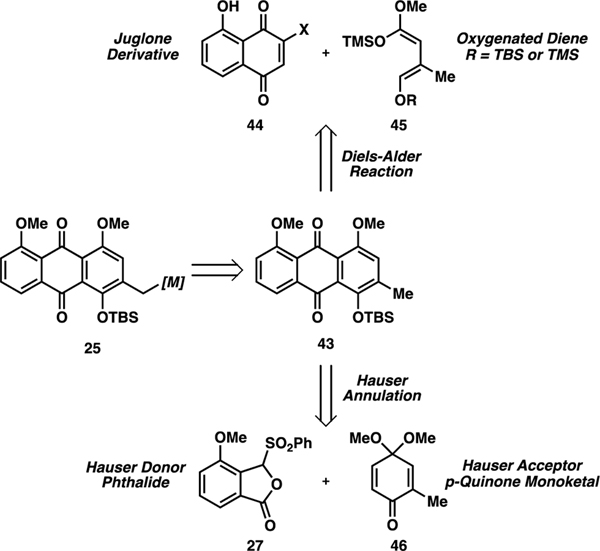
We devised a few strategies to access the novel multi-substituted anthraquinone core 43, which would then be metallated at its benzylic position to organometallic species 25 (Scheme 10). Since the anthraquinone ultimately has to undergo a late-stage Heck reaction (Scheme 3), one of the positions of anthraquinone 43 had to be alternatively functionalized (either through protecting group or halogenation) since a suitable C–H functionalization of this position did not seem feasible due to steric requirements and electronic competition. With this in mind, we devised a Diels–Alder approach30 and a Hauser annulation approach toward anthraquinone 43. In the Diels–Alder approach, a halogenated naphthoquinone derivative 44 could undergo a regioselective [4+2] cycloaddition with a properly designed diene 45; a requirement for successful aromatization would be retainment of an oxygenated substituent (OR) in diene 45. The Tietze group had previously showed that when R = TMS of diene 45, the silyl group was quickly hydrolyzed and a free alkoxy group was retained.30 Our goal was the preservation of the full silyl group. Aromatization could furnish the desired anthraquinone with the correct protecting group scheme and benzylic position ready for functionalization. An alternate proposal proceeded through a Hauser annulation of sulfone 27 and p-quinone monoketal 46.
Scheme 10.
Retrosynthesis of Anthraquinone 43
The diene for the Diels–Alder reaction was built from methyl crotonate (Scheme 11).31 A Henry reaction furnished nitro compound 50, which underwent a Nef reaction to yield acetal 51. Further hydrolysis furnished aldehyde 52, a suitable precursor for the silylated derivatives necessary for our proposed Diels–Alder reaction. Silylation to the resulting silyl enol ether 53 or 54 followed by enolization and trapping with TMSCl furnished the silyl ketene acetals 55 and 56 in high yields.
Scheme 11.
Forward Synthesis of Dienes 55 and 56
The halogenated naphthoquinone was constructed from commercially available dihydroxy naphthoquinone 57 (Scheme 12).32 Initial oxidation with oxygen and copper(I) chloride furnished juglone 58 through a [4+2] addition of singlet oxygen and fragmentation of the resulting endoperoxide, which could then be brominated in a two-step procedure to selectively yield 3-bromojuglone 59.33 As reported by the Tietze group, chlorojuglones retain the highest activity in Diels–Alder reactions, and so we subjected bromojuglone 59 to hydrochloric acid to undergo halogen exchange to chlorojuglone 60. In our Diels–Alder examinations, we also attempted reactions on the methylated chlorojuglone 61, available by methylation of 60.
Scheme 12.
Juglone Derivative Synthesis
The Diels–Alder reaction proved unsuccessful in retaining the correct protecting group and oxygenation schemes (Scheme 13). All attempts at cycloadditions with the tert-butyldimethylsilyl (TBS)-protected diene 56 gave elimination of the TBS-protected ether, including under various aromatization conditions, to produce small quantities of the natural product chrysophanol without our necessary element of oxygenation (62). We further attempted cycloadditions on the methylated juglone derivative 61 with hope that the altered electronics may allow for retention of the TBS-protected hydroxyl group in the aromatization stage. However, in all cases, these naphthoquinone derivatives were unreactive to the initial [4+2] cycloaddition due to the significant change in electronics and lack of internal hydrogen bonding of the dienophile 61. Finally, we mirrored the Diels–Alder reactions reported in the literature to access islandicin (63) with TMS-protected diene 55 and chlorojuglone 59. We attempted selective protections and deprotections of islandicin; however, the poor scale of this full reaction sequence, joined by the possibility of >5–10 steps of protecting group manipulation, made this route unattractive in the synthesis.
Scheme 13.
Diels–Alder Attempts and Further Anthraquinone Functionalizations of Islandicin
We simultaneously attempted a previously reported Hauser annulation approach to access the same anthraquinone intermediate (Scheme 14).34,35 A regioselective annulation would provide access to protected islandicin derivative 71, which could be further modified to meet our needs as a nucleophilic anthraquinone fragment. Hauser donor sulfone 27 was produced by an ortho-metalation strategy.36,37 3-Methoxybenzoic acid 64 was amidated, followed by directed ortho-formylation to aldehyde 66. Hydrolysis and sulfidation gave sulfide 68, which was oxidized to sulfone 27. Finally, o-cresol (69) was oxidized to Hauser acceptor 46.38 Upon combination of fragments in basic conditions, anthraquinone 71 was accessed through the regioselective annulation reaction (via intermediate 70).39
Scheme 14.
Hauser Annulation Approach39 to Anthraquinone 71
At the completion of anthraquinone 71, we quickly realized, rather than attempting to produce the unprecedented benzylic anthraquinone organometallic with all its potential drawbacks (e.g., ease if reduction in the presence of many metals, diminished nucleophilicity), we could instead utilize the Hauser annulation in a late-stage reaction and introduce the anthraquinone fragment through a benzylic nucleophile derived from o-cresol (69). This proved to be the major shift in the synthetic sequence; we had now shifted from an early-stage anthraquinone introduction strategy to a late-stage approach.
Third-Generation Approach: Model System and Completion of (+)-Rubellin C.
In our third-generation retrosynthetic strategy,18 we ultimately decided on installation of the anthraquinone at a late stage of the synthesis (Scheme 15). This would allow for the oxidatively labile, poorly soluble, and highly polar ring system to be installed through a Hauser annulation, avoiding the aforementioned problems. The anthraquinone moiety on compound 18 could be introduced by a Hauser annulation with a p-quinone monoketal (not shown) derived from aromatic oxidation of homoallylic lactone 72. We still held the reasoning that the key hindered ring fusion could be selectively accessed through allylboron species 73 and aldehyde 20 (see Scheme 3). The stereochemically enriched allylating reagent could be prepared from the aforementioned enone 24, synthesized during our pursuit of the second-generation synthetic strategy, and nucleophilic benzylic fragment 26 derived from o-cresol (69).
Scheme 15.
Third and Successful Retrosynthesis of (+)-Rubellin C
Our synthesis began with production of Grignard reagent 26 (Scheme 16). As we now sought to introduce the anthraquinone via a late-stage Hauser annulation, we had to install only a benzylic fragment onto the β position of enone 24. Bromide 75 was accessed expediently from o-cresol (69).40 After significant experimentation, Grignard reagent 26 could be prepared on large scale (40–50 g) and titrations (0.7–0.8 M, 1 M target) by placing the reaction vessel in an ice bath after initiation to control the exotherm of Grignard reagent formation. Temperature control was key to high titrations of the Grignard reagent; uncontrolled exotherms led to dimerization and lower yields (20–30%, 0.2–0.3 M). Various other organometallic reagents were also attempted, including potassium anions utilizing “super-base” protocols and organozinc species, as described by Knochel et al.; however, many of these approaches fell short of our target titration and yield.41 We hope that this simple method and alteration to Grignard procedures will prove useful in the synthesis of other organometallic species prone to dimerization.
Scheme 16.
Benzylic Grignard Reagent 26 Synthesis
Benzylic Grignard 26 underwent copper-mediated conjugate addition into enone 24 smoothly in the presence of TMSCl and TMEDA as a base, chelating, and solubilizing agent (Scheme 17). The resulting mixture containing the silyl enol ether was converted into substituted enone 76 through the Saegusa–Ito oxidation with stoichiometric palladium(II). Various protocols were attempted for this oxidation to regenerate the palladium(II) from palladium(0); however, none could rival the traditional use of stoichiometric palladium(II) acetate under oxygen atmosphere in yield or conversion.42 Hydride reduction of the enone 76 carbonyl center would presumably occur from the convex face as in the previously observed chemistries, illustrated in transition state 79. An allylic borylation would then give our desired stereochemistry by another invertive mechanism. After screening various reductants such as traditional hydride sources including NaBH4, LiAlH4, and Luche conditions, we found that L-selectride (lithium tri-sec-butylborohydride) gave the best chemo- and diastereoselectivity for addition into the re-face of bicyclic enone 76 on multigram scale to furnish allylic alcohol 77 (Scheme 17). To design our allylboron species 72 (Scheme 15), we focused on recently reported borylation chemistry from the Szabó group that stereospecifically converts chiral allylic alcohols into allylboron reagents.43 Inversion of alcohol 77 using bis(pinacolato)diboron (B2pin2) and a palladium(II) tetrafluoroborate catalyst Pd(MeCN)4(BF4)2 produced pinacol boronic ester 78 in high diastereoselectivity. Prolonged reaction times and higher catalyst loadings gave the opposite isomer due to the reactivity of excess palladium(0) with the intermediate palladium(II) complex.
Scheme 17.
Pinacol Allylboronic Ester 78 Synthesis
With a possible allyl nucleophile prepared, we first examined our stereochemical hypotheses in a model system of the rubellin core (Scheme 18). Thermal allylation of 78 with benzaldehyde furnished homoallylic alcohol 81 as one isolable diastereomer, presumably through energy-minimized transition state 80. This transformation installed the quaternary center at C16 and alcoholic stereocenter at C18 in both high yield and diastereoselectivity. The X-ray crystal structure of free phenol 82, obtained by removal of the TBS group, confirmed our stereochemical analysis in the preceding allylation, borylation, and reduction events. Triflation of phenol 82 and subjection to palladium(0) conditions gave one diastereomer of the final 6–5-6-fused system 85.44 This occurs through oxidative addition of the intermediate triflate onto the palladium(0) complex to form a palladium(II) species 83 oriented on the back face of the molecule due to the stereochemistry at the quaternary center. The carbopalladation is then directed by the quaternary center to set the C5–C11 carbon bond in the model system through a 5-exo cyclization to intermediate 84. The resulting palladium(II) intermediate then must undergo β-hydride elimination with a syn periplanar hydride, not present at C11 and only available at C13. This allows for installation of the alkene out of conjugation of the aromatized system in the core system 85. The stereochemistry was designated to be the cis-ring junction by nOe correlations as desired in the natural product scaffold.
Scheme 18.
Diastereoselective Completion of Rubellin Core 85
Optimization and Screening of Key Allylation.
After gaining supporting evidence for our stereochemical hypotheses, we began the allylation of aldehyde 20, available from commercial materials in seven steps via an ortho-lithiation strategy of a salicylic acid derivative 86 (Scheme 19).45 The same synthetic strategy was applied for Hauser donor 27 previously (Scheme 14); however, the resulting lactol here 90 was instead opened to the carboxylate and methylated to produce aldehyde 20.
Scheme 19.
Synthesis of Aldehyde 20
At this point, we began examining the reactivity of our initial allylating reagent 78 with aldehyde 20 (Scheme 20). Treatment of pinacol boronic ester 78 with aldehyde 20 gave no yield of the desired homoallylic lactone under harsh thermal conditions (>220 °C); only decomposition of the allylboron reagent was observed. Lewis acid or Brønsted acid additives decomposed the allylating reagent through protodeborylative processes with no appropriate reactivity observed.20,21 Ligand exchange with a fluoride source or alcohols to the potassium trifluoride boronate salt or smaller boronic esters progressed with no conversion, often epimerizing the carbon–boron bond or removing the silyl group.46,47 Esterification or halogenation of allylic alcohol 77 followed by the introduction of other metals known to proceed through type I allylation reactions provided low reactivity (<5% yield) with a mixture of isomers.48–50 In many cases, we observed scrambling of stereochemistry at the carbon–metal bond. We returned to boron, examining first the production of borinic esters from pinacol boronic ester 78.51 Treatment with organolithium reagents such as MeLi or nBuLi and trapping with trifluoroacetic anhydride (TFAA) gave various borinic esters, which did not allylate aldehyde 20 at low temperatures and were not thermally stable. All of these derivatives proved unsuccessful in formation, stereochemical fidelity during construction and in transfer of chirality, or in ability to facilitate the key carbon–carbon bond-forming event.
Scheme 20.
Attempted Allylations of Aldehyde 20
We then examined the formation of allylboronic acids instead of allylboronic esters from allylic alcohol 77 utilizing Pd(MeCN)4(BF4)2 and bisboronic acid B2(OH)4 as reported in an altered protocol from the Szabó group, utilizing benzaldehyde as an effective titrating agent.52 Allylboronic acids lack a hindered steric environment around the empty p-orbital of boron and are hypothesized to form remarkably Lewis acidic trimeric allylic boroxines upon removal of water, increasing their allylative reactivity.53 The trimeric allylboroxine has one less oxygen per boron than the corresponding allylboronic acid, greatly increasing the Lewis acidity of each boron atom in a planar array. Use of Pd(MeCN)4(BF4)2 with B2(OH)4 in a DMSO/MeOH mixture followed by oxygen-free extraction and dehydration with Na2SO4 gave postulated allylboron species 91 likely in equilibrium with its boroxine trimer counterpart, which underwent allylation with benzaldehyde to give the same diastereomer 81 as produced in the studies of the core system. A significant level of optimization from the originally reported conditions produced a viable solvent mixture of DMSO/MeOH to facilitate the two-step allylic borylation (full details can be found in the Supporting Information in Table S14).
With optimized conditions in hand, allylation of aldehyde 20 at a higher temperature gave homoallylic lactone 72 as one isolable diastereomer (Scheme 21). We realized in our studies that aldehyde 20 could be used as a limiting reagent to produce high yields of lactone 72 with recovery of starting allylic alcohol 77.
Scheme 21.
Two-Step Diastereoselective Transformation of Allylic Alcohol 77 to Lactone 72
The stereochemical control of this transformation stems from the stereochemistry at the hydroxyl stereocenter of allylic alcohol 77. Initial inversion of the BF3-activated hydroxyl bond (92) through oxidative addition of an in situ generated Pd0 species is followed by a rate-limiting transmetallation of B2(OR)4 for either the pinacol ester or bisboronic acid, as studied by the Szabó group.54 Reductive elimination of the carbon–boron bond of intermediate 94 occurs on the least substituted carbon and the convex face of the bicyclic system to generate allylboron species 95 (Scheme 22). The transmetallation of the boron–boron bond onto palladium is facilitated by its electron density; the rate of transmetallation is then inversely proportional to the Lewis acidity of the boron reagent used. Therefore, the boron reagents that are least successful in the palladium-catalyzed borylation will be most successful in the aldehyde allylation, as observed in the differences between bis(pinacolato)diboron and bisboronic acid in the two-step transformation.
Scheme 22.
Mechanism54 of Allylic Alcohol 77 Borylation and Subsequent Allylation
The stereochemistry of the allylation is controlled through the Zimmerman–Traxler chair-like transition state 96 to simultaneously set the quaternary and hydroxyl stereocenters in homoallylic lactone 72. This strategy allows for preparation of the Csp3–Csp3 ring junction with a high control of stereo- and regioselectivity in the generation of one out of eight possible constitutional and stereochemical isomers on gram scale.
Analysis of Hauser Annulation.
To complete the synthesis of rubellin C, we pursued the late-stage Hauser annulation, triflation, and Heck reaction (Scheme 23). Analogous removal of the silyl group of allylation product 72 with tetra-n-butylammonium fluoride (TBAF) provided the free phenol 97. Higher equivalents (>1.3) of TBAF epimerized the C18 lactone stereocenter, hinting at its lability and a problem that would have to be overcome later in the synthesis. Oxidation of the free phenol with (diacetoxyiodo)benzene formed the p-quinone monoketal 98 in a 60% yield over two steps.
Scheme 23.
Synthesis of Hauser Acceptor 98
Initial examination of the Hauser annulation with bases such as the dimsyl anion or lithium diisopropylamide and matching equivalents of sulfone 27 and p-quinone monoketal 98 gave low yields of one bright red anthraquinone isomer, visible without staining on thin-layer chromatography plates and during column chromatography as a red band.27 Switching to lithium tert-butoxide gave anthraquinone 18 in higher yields; however, with higher base equivalents (>3) or upon application of heat, scrambling of the C18 stereocenter was observed to give full conversion to undesired epimer 100 (Table 2). After optimization, the desired epimer 18 could be acquired with high yield and stereoretention with 2.65 equivalents of base, minimizing the formation of epimer 100, the structure of which was confirmed by X-ray crystallography. The crystal structure of the undesired epimer 100 further established our stereochemical assignment of the allylation preceding this step (Scheme 21, compound 72). If anthraquinone isomer 100 was desired, extra equivalents of base or heating of the system would provide it in full conversion. This likely occurs through deprotonation at C18 to form alkoxyfuran species 99. Protonation on the opposite face forms the opposite, thermodynamically favored isomer. This behavior of intermediates was not observed with later intermediates beyond the Heck reaction (vide infra), insinuating that once the key 6–5-6-fused system is put in place, epimerization at the C18 center is not as thermodynamically favored.
Table 2.
Optimization of Late-Stage Hauser Annulation

|
Reaction run on a 0.6 mmol scale to demonstrate scalability.
We also employed these tactics on a sulfone derivative of sulfone 27 toward (+)-rubellin D. Sulfone 106 was synthesized in the same manner as sulfone 27 from a dimethoxybenzoic acid derivative 101 in a similar sequence of amidation, o-formylation, hydrolysis, sulfidation, and oxidation (Scheme 24).
Scheme 24.
Synthesis of Hauser Donor 106 for (+)-Rubellin D
Hauser chemistries with sulfone 106 and previously discussed Hauser acceptor 91 furnished a 3:1 mixture of anthraquinone isomers 107 and 108 under the same conditions as developed for the rubellin C synthesis (Scheme 25). Desired isomer 107 was carried forward into the triflation and Heck chemistries.
Scheme 25.
Hauser Annulation for Precursor 107 to (+)-Rubellin D
Heck Reaction and Completion of (+)-Rubellin C.
With the Hauser conditions established for anthraquinone 18 as well as for rubellin D precursor 107, we pursued the final steps of the synthesis. Typical bases used in triflation conditions were not suitable for deprotonation of the chelated phenol proton; soluble bases also assisted with C18 epimerization. After considerable optimization, our conditions involved a long deprotonation with sodium hydride in dilute CH2Cl2, followed by the addition of trifluoromethanesulfonic anhydride to give access to triflate 109 (Table 3). This minimized epimerization and deprotection of the acetonide while generating reasonable yields of the desired triflate 109. During the course of the deprotonation, we observed a purple solid deposited on the side of the flask in significant quantities, which we hypothesize is the insoluble sodium salt of phenol 18 that cannot undergo epimerization due to its precipitation.
Table 3.
Optimization of Anthraquinone Triflation

| ||||||
|---|---|---|---|---|---|---|
| entry | solvent | base (equiv) | triflating reagent | temp (°C) | time (h) | 109 (% yield) |
| 1 | CH2Cl2 | Pyr (20) | PhNTf2 | 0–40 | 15 | 0 |
| 2 | CH2Cl2 | DBU (10) | PhNTf2 | 0–40 | 15 | 0 |
| 3 | THF | LDA (3) | PhNTf2 | −78–23 | 15 | 0 |
| 4 | CH2Cl2 | DBU (20) | Comins’ | 0–23 | 15 | 0 |
| 5 | CH2Cl2 | Pyr (20) | Comins’ | 0–23 | 15 | 0 |
| 6 | CH2Cl2 | Pyr (3) | Tf2O | −78–0 | 2 | 36 |
| 7 | THF | NaH (10) | Tf2O | −78 | 2 | 0a |
| 8 | CH2Cl2 | NaH (10) | Tf2O | 0 | 5 | 50 |
| 9 | DME | NaH (10) | Tf2O | 0 | 5 | 50b |
| 10 | CH2Cl2 | NaH (5) | Tf2O | 0 | 5 | 0 |
| 11 | CH2Cl2 | NaH (20) | Tf2O | 0 | 5 | 72% |
| 12 | CH2Cl2 | NaH (20) + Pyr (3) | Tf2O | 0 | 5 | 73%c |
The polymer of tetrahydrofuran (THF) produced by triflic anhydride (Tf2O) decomposes the mixture of product and starting material quickly during purification.
Mixture of isomers.
The deprotonation of anthraquinone 3 with sodium hydride is very slow on larger scales (>0.1 mmol). The addition of pyridine after Tf2O increased the conversion and reaction rate at a 0.23 mmol scale.
Triflate 109 was immediately used in the subsequent Heck reaction in a two-step protocol to present one diastereomer of the rubellin architecture (Scheme 26). Although a variety of bidentate phosphine ligands55 and different palladium(0) precursors were screened, the most reproducible results were simply with higher catalyst loadings of Pd(PPh3)4.56 Halide additives such as lithium bromide or lithium chloride were essential to the progression of the reaction to increase both polarity of the solvent media and the rate of oxidative addition of the aryl triflate.57 The major competing reaction in this process is detriflation of the intermediate triflate 109. This occurs in the presence of all halide salts to form a highly stable anthraquinonealkoxy anion. Nonetheless, reasonable yields of Heck product 110 were furnished under this two-step protocol in high diastereoselectivity through a similar mechanism as discussed in the model system (Scheme 18).
Scheme 26.
Two-Step Triflation and Heck Reaction of Compound 18
With the Heck reaction completed for the carbon framework of rubellin C (3), rubellin D (4) was pursued with anthraquinone 107 (Scheme 27). Although triflation under the developed conditions was successful, the resulting Heck reaction to compound 111 did not proceed, presumably due to the extra O-methyl ether present on the anthraquinone ring. This directly hampers the rate of oxidative addition to the triflate and allows for a competing detriflation to be the major reaction in the mixture, resulting in the recovery of phenol 107 and its epimer 108 in the two-step process.
Scheme 27.
Attempted Heck Reaction of (+)-Rubellin D Precursor 107
The final deprotection of the rubellin C (3) framework was attempted. A one-pot O-demethylation and acetonide removal of trimethylated substrate 110 were first attempted (Table 4). We began our screening with Lewis acidic demethylating agents; we would often observe the most electron-rich methyl group removed (C23 O) and then a significant decomposition event and cleavage of large sections of the molecule. We transitioned to nucleophilic demethylating agents to pursue a two-step deprotection procedure. These agents typically immediately removed the two electron-poor highly chelated methyl groups at C1 and C8 in a matter of minutes. After some screening, the weakly nucleophilic iodide source of freshly prepared magnesium iodide etherate (MgI2 OEt2) suitably removed all of the methyl groups at higher temperatures to give tri-demethylated product 112.58 Crucial to the success of this method was keeping the system in the dark; any superfluous iodine produced underwent SNAr reactions with the present aromatic rings, yielding mixtures of iodinated products.
Table 4.
Screening of Demethylation Conditions of Rubellin Precursor 110

| ||||||
|---|---|---|---|---|---|---|
| entry | solvent | reagent (equiv) | additive (equiv) | temp (°C) | time (h) | products observed |
| 1 | CH2Cl2 | BBr3 (10) | −78 | 1 h | C23 O-demethylation | |
| 2 | CH2Cl2 | BBr3 (10) | −78–0 | 2 h | decomposition | |
| 3 | CH2Cl2 | BBr3 (10) | −78 | 4 h | C23 O-demethylation; decomposition | |
| 4 | CH2Cl2 | BBr3 (3) | −78 | 15 | C23 O-demethylation | |
| 5 | CH2Cl2 | BBr3 (10) | −40 | 1 | C23 O-demethylation; decomposition | |
| 6 | CH2Cl2 | BBr3 (10) | amylene (11) | −40 | 1 | C23 O-demethylation; decomposition |
| 7 | None | Pyr-HCl (200) | 200 | 1 | decomposition | |
| 8 | CH2Cl2 | BCl3 (10) | 0 | 24 | C23 O-demethylation; decomposition | |
| 9 | CHCl3 | TMSl (4) | 0 | 0.5 | C1/8 O-demethylation; decomposition | |
| 10 | CH2Cl2 | AlCl3 (10) | 23 | 5 | mixture of demethylated epimers | |
| 11 | CH2Cl2 | AlCl3 (10) | EtSH (10) | 0 | 0.25 | C1/8 O-demethylation; decomposition |
| 12a | THF | MgI2·OEt2 (10) | 50 | 3 | C1/8 O-demethylation; epimerization | |
| 13 | PhMe | MgI2·OEt2 (12) | 100 | 2 | 112 major; not full conversion | |
| 14 | PhMe | MgI2·OEt2 (14) | 100 | 3 | 112 | |
Initial experiments with MgI2·OEt2 were allowed to cool for 30 min to 1 h before quenching. This gave epimerization of the C18 center as well as iodinated byproducts. Entries 13 and 14 were quenched within 10–15 min of removal from the heating oil bath.
Finally, removal of the acetonide under acidic conditions59,60 produced one enantiomer of the natural product (+)-rubellin C (3) (Scheme 28). Our characterization data matched that described in the literature, and our synthetic efforts therefore also confirm the absolute stereochemistry reported of natural (+)-rubellin C (3).
Scheme 28.
Final Two-Step Deprotection and Completion of (+)-Rubellin C (3)
CONCLUSIONS
This manuscript describes our synthetic efforts toward the rubellin family of natural products, culminating in the synthesis of (+)-rubellin C (3). The first total synthesis of a member of the rubellin family of natural products has been accomplished with high stereoselectivity in 16 steps (longest linear sequence) from the readily available D-(−)-quinic acid (21). The expedient construction of the core ring system relied on an efficient sequence of steps to install topological and stereochemical complexity. This synthesis displays the current power of organic synthesis to access some of the most complex anthraquinone-containing natural products discovered to date with high stereochemical control. Further studies of these transformations, compounds, and their biological activities are in progress.
EXPERIMENTAL SECTION
All reactions were performed in flame-dried round-bottom flasks capped with septa, 8 mL capped reaction vials, or 4 mL capped reaction vials with magnetic stirring under a positive pressure of argon gas and with typical Schlenk line techniques unless otherwise indicated. Commercially obtained reagents were used as received. Solvents were dried by passage through an activated alumina column under argon. Liquids and solutions were transferred via a syringe and a stainless steel needle. All reactions were monitored by thin-layer chromatography with E. Merck silica gel 60 F254 precoated plates (0.25 mm) unless otherwise indicated and were visualized by UV (254 nm) and/or KMnO4 staining. Silica gel (particle size 0.032–0.063 mm) purchased from SiliCycle was used for flash chromatography unless otherwise specified. 1H and 13C NMR spectra were recorded on Varian Inova-500 (500 MHz) spectrometers, Varian Inova-400 (400 and 90 MHz, respectively) spectrometers, Bruker 400 (400 and 101 MHz, respectively) spectrometers, or Bruker 600 (600 and 151 MHz, respectively) spectrometers. Data for 1H NMR spectra are reported relative to chloroform (7.26 ppm), benzene (7.16 ppm), or dimethyl sulfoxide (2.50 ppm) as an internal standard and are reported as follows: chemical shift (δ ppm), multiplicity, coupling constant (Hz), and integration. Data for 13C NMR spectra are reported relative to chloroform (77.0 ppm), benzene (128.1 ppm), or dimethyl sulfoxide (39.5 ppm) as an internal standard in terms of chemical shift (δ ppm). Infrared spectra were recorded on a Mettler Toledo ReactIR 15 with a DST Series 6.3 mm AgX FiberCoduit Au/Silicon Probe. HRMS data (ESI) were obtained at the UT Southwestern Metabolomics Core Facility on an SCIEX TripleTOF 6600 High-Resolution Accurate Mass System and the Shimadzu Center for Advanced Analytical Chemistry (SCAAC) at UT Arlington. ESI/APCI-LRMS data were recorded on an AB Sciex QTRAP 4500 LCMS. Optical rotations were measured on a JAS DIP-360 digital polarimeter. X-ray diffraction data were obtained from Dr. Vincent Lynch at the X-ray Diffraction Lab at The University of Texas at Austin.
Compound 30.
Prepared as previously reported with D-(−)-quinic acid (57.6 g, 300 mmol, purchased from Sigma-Aldrich in 100 g bottles, CAS 77–95-2), p-toluenesulfonic acid monohydrate (656 mg, 3.45 mmol), 2,2-dimethoxypropane (115 mL, 942 mmol), and EtOAc (Sigma-Aldrich, HPLC grade, >99.8%, 402 mL) to give lactol 30 as white needles (46.6 g, 216.5 mmol, 72%).18,22
Compound 31.
Prepared as previously reported with THF (150 mL, 0.27 M), LiAlH4 (4.60 g, 120 mmol, 3 equiv), and lactol 30 (8.6 g, 40 mmol) in THF (50 mL, 0.8 M) to give triol 31 as a white solid (6.8 g, 78%).22
Compound 32.
Prepared as previously reported with NaIO4 (19.1 g, 89.4 mmol, 1.5 equiv), H2O (42.1 mL), silica gel (90.0 g), and compound 31 (13.0 g, 59.6 mmol) to give hydroxyketone 32 (11.0 g, 99%).23,24
Compound 33.
Prepared as previously reported with hydroxyketone 32 (3.2 g, 17 mmol), MeOH (21.25 mL, 0.8 M), and NaBH4 (646 mg, 17 mmol) to give compound 33 (1.92 g, 60%).23
Compound 29.
Prepared as previously reported with diol 33 (1.92 g, 10.2 mmol), pyridine (15.7 mL, 0.65M), DMAP (378 mg, 3.1 mmol, 30 mol %), and p-TsCl (1.94 g, 10.2 mmol, 1 equiv) to give tosylate isomers 34 and 35 (2.13 g, 61%), which were immediately used in the next reaction with oxalyl chloride (1.34 mL, 15.6 mmol, 2.5 equiv), CH2Cl2 (80 mL), DMSO (5.50 mL, 77.6 mmol, 12.5 equiv), and NEt3 (10.7 mL, 77.6 mmol, 12.5 equiv) to give enone 29 (614 mg, 59%).23
New: Compound 28.
A flame-dried 2-neck 25 mL round-bottom flask was charged with CuCl (109 mg, 1.1 mmol, 1.1 equiv) and oven-dried LiCl (47 mg, 1.1 mmol, 1.1 equiv). DMF (3 mL) was added to the solids, and the mixture was stirred for 1 h. At this time, bis(pinacolato)-diboron (280 mg, 1.1 mmol, 1.1 equiv), KOAc (108 mg, 1.1 mmol, 1.1 equiv), and enone 29 (168 mg, 1 mmol) in DMF (3 mL) were added sequentially. The reaction was brought to 60 °C for 15 h. After cooling, the reaction was quenched with H2O and extracted with Et2O (5×, total V = 50 mL). The organic layers were dried over MgSO4, filtered, and concentrated to a residue. The title compound 28 was purified by flash column chromatography (50% EtOAc/hexanes) to give the borylated product as a colorless semisolid (97 mg, 33%). 1H NMR (500 MHz, CDCl3): δ 4.61 (dt, J = 5.7, 2.9 Hz, 1H), 4.25 (d, J = 5.3 Hz, 1H), 2.51 (dt, J = 14.2, 2.7 Hz, 1H), 2.29–2.19 (m, 2H), 1.93 (ddd, J = 15.4, 13.1, 3.4 Hz, 1H), 1.74 (t, J = 13.5 Hz, 1H), 1.42 (s, 3H), 1.38 (s, 3H), 1.23 (s, 12H). 13C NMR (101 MHz, CDCl3): δ 208.5, 109.8, 83.8, 78.0, 77.4, 41.2, 28.6, 27.2, 26.2, 25.0, 24.8, 24.8, 24.8, 24.8. 11B NMR (128 MHz, CDCl3): δ 33.33. FTIR (ReactIR): 1750, 1383, 1331, 1143, 854. LCMS (ESI) m/z: calculated for [M + Na]+ 319.18, found 319.2. (c = 0.15, CHCl3).
Magnetic Catalyst Fe3O4•Cu(OH)x Preparation.
Prepared as previously reported with CuCl2 (130 mg, 1 mmol), Fe3O4 (<5 μM particles, 4 g, 17 mmol), and H2O (120 mL). The black powder was magnetic and usable in borylation reactions for over a month as described in its preparation.25
New: Compound 28.
A flame-dried vial was charged with bis(pinacolato)diboron (356 mg, 1.4 mmol, 1.4 equiv) and toluene (1 mL, 1 M). The iron/copper catalyst (100 mg), K2CO3 (124 mg, 0.9 mmol, 0.9 equiv), enone 29 (168 mg, 1 mmol), and MeOH (81 μL, 2.0 mmol, 2.0 equiv) were added. The vial was submerged in a 60 °C oil bath for 2 h. After cooling, the reaction was quenched with sat. NH4Cl aqueous solution and extracted with Et2O (5×, total V = 50 mL). The organic layers were dried over MgSO4, filtered, and concentrated to a residue. The title compound was purified by flash column chromatography (50% EtOAc/hexanes) to give the borylated product 28 as a colorless semisolid (250 mg, 84%).
New: Compound 36 and Triflation Protocol for Table 1.
Borylated enone 28 (60 mg, 0.2 mmol) was treated with a solvent (0.2 M), a base (1.1–excess equiv) at the indicated temperature, and a triflating reagent (1 equiv–excess) under argon (see Table 1). The major product in all cases observed was undesired vinyl triflate 36 as a clear oil (varied yields from 0 to 85%; see Table 1 in the text). 1H NMR (500 MHz, CDCl3): δ 4.73–4.66 (m, 1H), 2.55 (q, J = 2.9 Hz, 2H), 2.36 (ddd, J = 11.6, 5.3, 3.2 Hz, 1H), 1.77–1.70 (m, 1H), 1.54 (s, 3H), 1.47 (s, 3H), 1.23 (s, 13H). 13C NMR (101 MHz, CDCl3): δ 143.1, 121.0, 120.2, 116.9, 114.2, 84.0, 73.3, 28.4, 28,4, 27.1, 27.1, 26.9, 24.8, 24.8, 24.5, 24.5. 19F NMR (400 MHz, CDCl3): δ 74.41. FTIR (ReactIR): 1752, 1413, 1380, 1329, 1215. LCMS (ESI) m/z: calculated for [M + H]+ 429.12, found 429.1. (c = 0.45, CHCl3).
Compound 50.
Prepared as previously reported with starting material 49 (10.65 mL, 100 mmol), nitromethane (27 mL, 500 mmol, 5 equiv), and tetramethylguanidine (2.5 mL, 10 mmol, 10 mol %) to give nitro compound 50 (13.5 g, 84%).31
Compound 51.
Prepared as previously reported with NaOMe from sodium (1.13 g, 49.5 mmol) and MeOH (33.1 mL) and compound 50 (7.27 g, 45 mmol) to give acetal 51 (5.80 g, 73%).31
Compound 52.
Prepared as previously reported with H2O (11 mL, 3 M) and crude acetal 51 (5.80 g, 33 mmol) to give compound 52 (3.43 g, 80%).31
Compound 53.
Prepared as previously reported with ZnCl2 (158 mg, 1.16 mmol, 3 mol %), NEt3 (11.8 mL, 85 mmol, 2.2 equiv), aldehyde 52 (5.02 g, 38.6 mmol), dry benzene (11.6 mL, 3.33 M), and TMSCl (9.8 mL, 77.5 mmol, 2 equiv) to give compound 53 (6.20 g, 79%).31
New: Compound 54.
Compound 52 (260 mg, 2 mmol) was dissolved in dry CH2Cl2 (20 mL, 0.1 M) and cooled to 0 °C. TBSOTf (920 μL, 4 mmol, 2 equiv) and NEt3 (552 μL, 4 mmol, 2 equiv) were added sequentially, and then the reaction was allowed to come to room temperature overnight. At this time, the reaction was treated with water, and the layers were separated. The aqueous layer was washed with CH2Cl2 (3×). The organic layers were compiled, washed with brine, dried over Na2So4, filtered, and concentrated. The title compound was purified by flash column chromatography (5% EtOAc/hexanes) to afford a clear oil (300 mg, 82%). 1H NMR (500 MHz, CDCl3): δ 6.20–6.10 (m, 1H), 3.67 (s, 3H), 2.86 (s, 2H), 1.65 (d, J = 1.5 Hz, 3H), 0.92 (s, 11H), 0.12 (s, 6H). 13C NMR (101 MHz, CDCl3): δ 171.9, 137.7, 111.2, 51.1, 39.2, 25.8, 25.8, 13.4, −5.3. FTIR (ReactIR): 1737, 1162, 842, 737, 716. LCMS (ESI) m/z: calculated for [M + Na]+ 267.15, found 267.2.
Compound 55.
Prepared as previously reported with LDA from i-Pr2NH (4.7 mL, 33.7 mmol, 1.1 equiv), THF (30.6 mL), n-BuLi (2.5 M in hexanes, 13.5 mL, 33.7 mmol, 1.1 equiv), TMSCl (5.8 mL, 46 mmol, 1.5 equiv), and silyl enol ether 53 (6.20 g, 30.6 mmol) in THF (8.7 mL) to give silyl ketene acetal 55 (7.63 g, 91%).31
New: Compound 56.
LDA was prepared by adding n-BuLi (2.5 M in hexanes, 540 μL, 1.35 mmol, 1.1 equiv) to a THF solution (1.23 mL) of diisopropylamine (189 μL, 1.35 mmol, 1.1 equiv) at −78 °C and subsequently mixing at −78 °C for 2 h. At this time, TMSCl (234 μL, 1.85 mmol, 1.5 equiv) was added to the LDA, and then compound 54 was added dropwise. The mixture was stirred at −78 °C for 15 min and then warmed to room temperature. The mixture was concentrated and diluted in hexanes and then poured over a short celite plug. The resulting clear liquid (317 mg, 82%) was immediately used in subsequent Diels–Alder reactions due to its instability. 1H NMR (500 MHz, CDCl3): δ 6.25 (dd, J = 1.5, 0.8 Hz, 1H), 4.24 (d, J = 0.7 Hz, 1H), 3.53 (s, 3H), 1.78 (d, J = 1.4 Hz, 3H), 0.92 (s, 12H), 0.24 (s, 9H), 0.11 (s, 6H). 13C NMR (101 MHz, CDCl3): the compound quickly protonates to 53 in various NMR solvents, and thus no 13C spectra were obtained. FTIR (ReactIR): 1700, 1681, 1651, 1622, 1060. LCMS (ESI) m/z: calculated for [M + Na]+ 339.19, found 339.2.
Juglone Synthesis (58).
Prepared as previously reported with four portions of CuCl (1.5 g, 15 mmol, 1 equiv each), MeCN (100 mL), O2 gas, and four portions of dihydroxynaphthalene 57 (2.5 g, 15 mmol each, purchased from Sigma-Aldrich in 100 g bottles, CAS 83–56-7) in MeCN (100 mL) to give compound juglone 58 (5.45 g, 52%).32
Compound 59.
Prepared as previously reported with juglone (58) (5.2 g, 30 mmol), AcOH (50 mL, 0.6 M), bromine (1.77 mL, 33 mmol, 1.1 equiv) and then with EtOH (15 mL, 2 M) to give compound 59 (6.20 g, 83%).32,33
Compound 60.
Prepared as previously reported with bromojuglone 59 (6.20 g, 24.5 mmol), EtOH (490 mL, 0.05 M), and HCl (61 mL, 0.4 M) to give compound 60 (4.31 g, 84%).33
Compound 61.
Prepared as previously reported with chlorojuglone 60 (104 mg, 0.5 mmol), Ag2O (231 mg, 1 mmol, 2 equiv), CHCl3 (5 mL, 0.1 M), and MeI (93.4 μL, 1.5 mmol, 3 equiv) and then Ag2O (115 mg) and MeI (62 μL) to give compound 61 (68 mg, 61%).30
General Procedure for Diels–Alder Attempts of Juglone Derivatives (59–61) and Dienes (55–56) (Scheme 13).30
Juglone (1 equiv) and diene (4 equiv) were added to a flask, followed by dry benzene. The reaction was stirred at room temperature or brought to reflux until the loss of both starting materials by TLC (3–15 h). The reaction was concentrated and redissolved in THF with a basic (e.g., NEt3) or acidic (e.g., HCl) additive to rearomatize the intermediate Diels–Alder adduct.
Synthesis of Islandicin (Compound 63).
Prepared as previously reported with 3-chlorojuglone 59 (1.45 g, 6.95 mmol), diene 55 (7.63 g, 27.8 mmol, 4 equiv) in benzene (35 mL), followed by THF (70 mL) and concentrated HCl (14 mL) to give islandicin 63 (1.77 g, 94%).30
Compound 65.
Prepared as previously reported with 3-methoxybenzoic acid 64 (7.61 g, 50 mmol) and thionyl chloride (8 mL, 110 mmol, 2.2 equiv) and then CH2Cl2 (38 mL, 1.3 M) and Et2NH (10.34 mL, 100 mmol, 2 equiv) to give compound 65 (9.21 g, 89%).36
Compound 66.
Prepared as previously reported with compound 65 (9.21 g, 44.4 mmol), TMEDA (6.66 mL, 44.4 mmol, 1 equiv), Et2O (111 mL, 0.4 M), sBuLi solution (1.4 M in cyclohexane, 33.3 mL, 46.6 mmol, 1.05 equiv), and DMF (4.14 mL, 53.3 mmol, 1.2 equiv) to give compound 66 (4.58 g, 43%).36
Compound 67.
Prepared as previously reported with compound 66 (2.90 g, 12.3 mmol), AcOH (glacial, 31 mL), and HCl (10%, 31 mL) to give compound 67 (1.78 g, 80%).36
Compound 68.
Prepared as previously reported with lactol 67 (180 mg, 1 mmol), p-toluenesulfonic acid hydrate (8.6 mg, 0.05 mmol, 5 mol %), MgSO4 (2 g, 1 g per mmol SM), toluene (5 mL, 0.2 M), and thiophenol (84 μL, 0.9 mmol, 0.9 equiv) to give compound 68 (259 mg, 95%).37
Compound 27.
Prepared as previously reported with sulfide 68 (259 mg, 0.95 mmol), CH2Cl2 (9.5 mL, 0.1 M), and two portions of m-chloroperoxybenzoic acid (<77%, 328 mg, 1.9 mmol, 2 equiv; then 164 mmol, 0.95 mmol, 1 equiv) to give compound 27 (268 mg, 0.88 mmol, 93%).37
Compound 46.
Prepared as previously reported with o-cresol (69) (515 μL, 5 mmol), MeOH (25 mL), and PhI(OAc)2 (354 mg, 11 mmol, 2.2 equiv) in MeOH (25 mL) to give compound 46 (400 mg, 48%).38
Compound 71.
Prepared as previously reported with LDA from i-Pr2NH (80 μL, 0.57 mmol, 1.2 equiv), n-BuLi (2.5 M in hexanes), and THF (2 mL) and then sulfone 27 (146 mg, 0.48 mmol) and p-quinone monoketal 46 (96 mg, 0.57 mmol, 1.2 equiv) in THF (2 mL) to give 71 as a red solid (102 mg, 71%).39
Compound 24.
Prepared as previously reported with compound 32 (11.0 g, 59 mmol), CH2Cl2 (333 mL, 0.18 M), triethylamine (24.5 mL, 177 mmol, 3 equiv), and methanesulfonyl chloride (5.5 mL, 70.8 mmol, 1.2 equiv) in CH2Cl2 (60 mL) to give enone 24 (8.13 g, 82%).24
Compound 74.
Prepared as previously reported with o-cresol 73 (15.5 mL, 150 mmol), DMF (150 mL, 1 M), tert-butyldimethylsilyl chloride (24.8 g, 165 mmol, 1.1 equiv), and imidazole (22.4 g, 330 mmol, 2.2 equiv) to give TBS-protected phenol 74 as a clear oil (33.3 g, 149 mmol, 99%).40
Compound 75.
A flame-dried 500 mL 2-neck flask and a condenser (note: a long condenser relative to the flask size was used, as the reaction is exothermic upon initiation) were charged with N-bromosuccinimide recrystallized from H2O (10 g per 100 mL, 26.5 g, 149 mmol) and azobisisobutyronitrile (3.67 g, 22.35 mmol). Benzene (Sigma-Aldrich, ACS Reagent Quality, 298 mL, 0.5 M) was added, followed by TBS-protected cresol 74. The mixture was degassed with a flow of argon for 30 min and then submerged into an 80 °C oil bath. After 2 h, the flask was removed from the oil bath and allowed to cool. During this period, succinimide precipitated on the walls of the flask. The reaction was diluted with hexanes (100 mL) and passed over a celite plug (2 CVs, hexanes). The resulting liquid was concentrated to an oil, which was purified through a silica gel plug (2 CVs, hexanes) to furnish bromide 75 as a clear oil (44.0 g, 99%). Spectral data matched the reported literature values.18,401H NMR (500 MHz, CDCl3): δ 7.33 (dd, J = 7.5, 1.8 Hz, 1H), 7.18 (td, J = 7.7, 1.7 Hz, 1H), 6.92 (td, J = 7.5, 1.1 Hz, 1H), 6.81 (d, J = 8.7 Hz, 1H), 4.54 (s, 3H), 1.05 (s, 10H), 0.29 (s, 6H).
Compound 26.
A flame-dried 250 mL 2-neck flask and a condenser were charged with magnesium powder (17.5 g, 730 mmol), iodine (100 mg), and THF (146 mL, 1 M). Benzyl bromide 75 (44.0 g) was added to the mixture, which was vigorously stirred. Initiation of the Grignard occurred between 5 and 15 min at room temperature with an initial color change from brown to colorless and an exotherm. Upon initiation, the reaction was immediately immersed into an ice bath and stirred for 1 h at 0 °C. After 1 h, the cold Grignard reagent 26 was titrated into salicylaldehyde phenylhydrazone (12.2 mg, 0.057 mml) in THF (1 mL) to afford a titration between 0.68 and 0.78 M (68–78% yield) and was immediately used in the next reaction (see synthesis of 76).18
Compound 76.
A 500 mL 2-neck flask with a stir bar was charged with copper(I) iodide (9.14 g, 95 mmol, 1.6 equiv) and flame-dried under vacuum. The flask was allowed to cool under vacuum and then blanketed with argon. THF (150 mL, 0.2 M) was added, and the mixture was cooled to 0 °C. Freshly prepared Grignard reagent 26 (53 mL, 0.68 M, 3.15 equiv) was added dropwise to the cooled solution, during which a black precipitate was observed. The mixture was stirred for 30 min at 0 °C. At this time, the flask was transferred to a dry ice/acetone bath and cooled to −78 °C. During cooling, tetramethylenediamine (TMEDA) (7.15 mL, 48 mmol, 1.6 equiv) was added dropwise. Once cooled to −78 °C, the mixture was charged with enone 24 (5.04 g, 30 mmol) and TMSCl (6.1 mL, 48 mmol, 1.6 equiv) in THF (48 mL) dropwise. The deep red mixture was stirred for 3 h at −78 °C. After completion of the 1,4-addition at −78 °C, the flask was placed in an ice bath at 0 °C for 1 h. Hexanes (50 mL) and EtOAc (50 mL) were added to the flask sequentially, followed by careful addition of aqueous sat. NaHCO3. The mixture was filtered, and the filtrate was poured into a separatory funnel. The aqueous layer was separated and extracted (×3, 50% EtOAc/hexanes, total V = 800 mL). The organic layers were compiled, washed with aqueous sat. NaHCO3, water (×3), and brine. The organic layers were dried over Na2SO4, filtered, and concentrated. The residue was placed onto a silica plug (3 CVs, 50% EtOAc/hexanes), and the resulting liquid was concentrated to afford a crude mixture of the intermediate silyl enol ether. The silyl enol ether was dissolved in DMSO (268 mL, 0.1 M) with vigorous stirring. Palladium(II) acetate (6.9 g, 30.8 mmol, 1.03 equiv) was added, and the reaction was stirred open to air at room temperature for 2 h. At this time, the reaction was diluted with EtOAc (200 mL) and filtered over a long celite pad. The resulting red liquid was washed with aqueous sat. NaHCO3, water (×3), and brine. The organic layers were dried over Na2SO4, filtered, and concentrated to a residue, which was purified by column chromatography (15% EtOAc/hexanes) to yield the title substituted enone 76 as a yellow oil (8.13 g, 78%).18 TLC (5:1 Hexanes:EtOAc). Rf 0.28 (UV, KMnO4). 1H NMR (400 MHz, CDCl3): δ 7.17–7.08 (m, 2H), 6.90 (td, J = 7.4, 1.2 Hz, 1H), 6.82 (ddd, J = 377.6, 8.0, 1.2 Hz, 1H), 5.67 (s, 1H), 4.64 (ddd, J = 5.1, 4.0, 2.7 Hz, 1H), 4.58 (dd, J = 5.0, 1.0 Hz, 1H), 3.72 (d, J = 16.4 Hz, 1H), 3.58 (d, J = 16.7 Hz, 1H), 2.86 (dd, J = 17.4, 2.8 Hz, 1H), 2.60 (dd, J = 17.4, 4.0 Hz, 1H), 1.41 (d, J = 2.0 Hz, 7H), 0.97 (s, 11H), 0.23 (d, J = 5.4 Hz, 7H). 13C NMR (101 MHz, CDCl3): δ 195.6, 159.4, 154.1, 131.5, 128.3, 127.1, 126.3, 121.4, 118.8, 109.9, 73.5, 73.3, 39.1, 34.3, 27.9, 26.8, 25.9, 18.4, −3.9, −3.9. FTIR (ReactIR): 1678, 1491, 1372, 1062, 839. HRMS (ESI) m/z: [M + H]+ calc. for C22H33O4Si 389.2143; found 389.2143. (c = 5.0, MeOH).18
Compound 77.
A flame-dried 500 mL flask was charged with THF (180 mL, 0.12 M) and L-selectride (1 M solution in THF, 26.1 mL) and cooled to −78 °C. Enone 76 (8.13 g, 20.9 mmol) was added dropwise in THF (29 mL, 0.72 M), and the reaction was stirred for 1 h at −78 °C. Aqueous sat. NH4Cl (26.1 mL) was added dropwise, and the mixture was allowed to warm to room temperature. The mixture was poured into a separatory funnel, and H2O (40 mL) and EtOAc (40 mL) were added. The layers were separated, and the aqueous layer was extracted (×3, EtOAc, final V = 300 mL). The organic layers were dried over Na2SO4, filtered, and concentrated to a residue, which was purified by flash column chromatography (5–10% EtOAc/hexanes) to yield the allylic alcohol 77 as a colorless oil (6.70 g, 82%).18 TLC (3:1 hexanes:EtOAc): Rf 0.28 (UV, KMnO4). 1H NMR (400 MHz, CDCl3): δ 7.13–7.01 (m, 2H), 6.88 (td, J = 7.4, 1.2 Hz, 1H), 6.80 (dd, J = 7.9, 1.2 Hz, 1H), 5.58 (d, J = 5.1 Hz, 1H), 4.51–4.38 (m, 1H), 4.33 (d, J = 5.3 Hz, 1H), 4.06 (q, J = 4.4 Hz, 1H), 3.56 (d, J = 15.6 Hz, 1H), 3.37 (d, J = 15.6 Hz, 1H), 2.35–2.21 (m, 1H), 1.94 (ddd, J = 14.8, 4.6, 2.6 Hz, 1H), 1.49 (s, 3H), 1.37 (s, 3H), 0.99 (s, 10H), 0.23 (s, 3H), 0.22 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 154.1, 137.6, 131.4, 129.0, 127.5, 127.0, 121.2, 118.7, 109.7, 74.2, 73.8, 63.8, 33.6, 32.4, 28.3, 26.7, 25.9, 18.4, −3.9, −4.0. FTIR (ReactIR): 2931, 1492, 1384, 1253, 1059. HRMS (ESI) m/z: [M + Na]+ calc. for C22H34O4SiNa 413.2119; found 413.2130. (c = 10.0, MeOH).
Compound 78.
A flame-dried 25 mL flask was charged with Pd(MeCN)4(BF4)2 (71 mg, 0.16 mmol, 10 mol %) and B2(pin)2 (488 mg, 1.92 mmol, 1.2 equiv). DMSO (2.15 mL) was added to the solids under argon to give an orange mixture. The allylic alcohol substrate 77 (625 mg, 1.6 mmol) was added in MeOH (2.15 mL). The mixture was stirred at room temperature, slowly turning red, then purple, and then black over 2 h. Methanol was removed on a rotary evaporator at 25 °C. The mixture was treated with Et2O and water and extracted with Et2O (×3, final V = 50 mL). The organic layers were dried on MgSO4, filtered, and concentrated to a residue, which was purified by flash column chromatography (10% EtOAc/hexanes) to give the allylboronic acid pinacol ester 78 as a colorless solid (400 mg, 51% yield).18 TLC (3:1 hexanes:EtOAc): Rf 0.71 (UV, KMnO4). 1H NMR (400 MHz, CDCl3): δ 7.15 (dd, J = 7.5, 1.8 Hz, 1H), 7.06 (td, J = 7.7, 1.8 Hz, 1H), 6.86 (td, J = 7.4, 1.2 Hz, 1H), 6.78 (d, J = 8.0 Hz, 1H), 5.56 (d, J = 3.5 Hz, 1H), 4.30 (td, J = 6.3, 3.5 Hz, 1H), 4.26 (d, J = 6.4 Hz, 1H), 3.59 (d, J = 15.7 Hz, 1H), 3.31 (d, J = 15.5 Hz, 1H), 2.01 (s, 1H), 1.89 (ddd, J = 13.4, 6.7, 5.1 Hz, 1H), 1.77 (ddd, J = 13.4, 7.8, 3.5 Hz, 1H), 1.42 (s, 3H), 1.35 (s, 3H), 1.20 (d, J = 1.6 Hz, 11H), 1.01 (s, 9H), 0.22 (s, 3H), 0.22 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 154.1, 133.9, 130.9, 130.3, 127.7, 127.0, 120.9, 118.7, 108.3, 83.4, 73.5, 34.0, 28.2, 27.4, 26.8, 26.0, 24.9, 24.8, 18.4, −3.9, −4.0. 11B NMR (128 MHz, CDCl3): δ 33.1. FTIR (ReactIR): 1492, 1451, 1372, 1253, 921. HRMS (ESI) m/z: [M + Na]+ calc. for C28H45O5SiBNa 523.3022; found 523.3040. (c = 1.0, CHCl3).
Compound 81.
An oven-dried microwave vial was charged with allylboronic ester 78 (230 mg, 0.46 mmol) as a solution in chloroform, which was sealed with a crimp cap and placed on a high vacuum on a Schlenk manifold for at least 3 h to remove the solvent. Toluene (4.6 mL, 0.1 M) was added under argon, followed by benzaldehyde (94 μL, 0.92 mmol, 2 equiv), and the sealed microwave vial was submerged in a 110 °C oil bath for 48 h. The reaction was then cooled, transferred to a 25 mL flask, and concentrated on a rotary evaporator. The resulting residue was taken up in EtOAc and saturated aqueous NH4Cl. The aqueous layer was extracted with EtOAc (3×, final V = 50 mL). The organics were washed with brine, dried over Na2SO4, filtered, and concentrated. Analysis of the crude extract showed one diastereomer of homoallylic alcohol product 13. The title compound 81 was purified by flash column chromatography (50% CH2Cl2/hexanes) to a colorless oil (163 mg, 74%).18 TLC (5:1 hexanes:EtOAc): Rf 0.43 (UV, KMnO4). 1H NMR (400 MHz, CDCl3): δ 7.70 (dd, J = 7.7, 1.8 Hz, 1H), 7.22–7.11 (m, 6H), 7.01 (td, J = 7.5, 1.3 Hz, 1H), 6.93 (dd, J = 8.1, 1.3 Hz, 1H), 5.80 (dq, J = 10.4, 1.8 Hz, 1H), 5.72 (dt, J = 10.3, 3.7 Hz, 1H), 4.31 (d, J = 3.8 Hz, 1H), 4.21 (d, J = 3.8 Hz, 1H), 3.78 (dd, J = 5.0, 1.4 Hz, 1H), 3.33–3.18 (m, 2H), 2.83 (dt, J = 8.4, 4.9 Hz, 1H), 1.78 (dddd, J = 18.6, 4.7, 3.5, 2.2 Hz, 1H), 1.59–1.48 (m, 2H), 1.43 (s, 3H), 1.40 (s, 3H), 1.05 (s, 9H), 0.30 (s, 3H), 0.25 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 154.2, 140.4, 132.5, 128.6, 128.4, 128.3, 127.9, 127.8, 127.6, 126.4, 122.5, 120.0, 106.7, 74.7, 49.0, 33.1, 30.4, 28.5, 26.1, 26.1, 18.6, −3.6, −4.2. FTIR (ReactIR): 2968, 1488, 1246, 1048, 914, 828. HRMS (ESI) m/z: [M + H]+ calc. for C29H41O4Si 481.2769; found 481.2788. (c = 0.5, CHCl3).
Compound 82.
A flame-dried 25 mL flask was charged with TBS-protected phenol 80 (150 mg, 0.31 mmol) in THF (3.1 mL, 0.1 M). After cooling to −78 °C, tetra-n-butylammonium fluoride (1 M in THF, 620 μL, 0.62 mmol, 2 equiv) was added dropwise, and the reaction was stirred at −78 °C for 45 min. The reaction was quenched by a 1:1 V/V water:saturated aqueous NaHCO3 solution and extracted with CH2Cl2 (×3, final V = 30 mL). The organic layers were dried over MgSO4, filtered, and concentrated to a solid residue, which was purified by flash column chromatography (20% EtOAc/hexanes) to yield the free phenol 82 as a white solid (107 mg, 94%).18 TLC (5:1 hexanes:EtOAc): Rf 0.13 (UV, KMnO4). 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H), 7.57 (dd, J = 8.1, 1.7 Hz, 1H), 7.39–7.23 (m, 5H), 7.19 (td, J = 7.6, 1.7 Hz, 1H), 6.89 (t, J = 7.0 Hz, 2H), 5.77 (dddd, J = 12.1, 10.4, 7.7, 2.7 Hz, 2H), 4.60 (s, 1H), 3.84 (dd, J = 5.2, 1.4 Hz, 1H), 3.23 (q, J = 14.3 Hz, 2H), 2.98 (ddd, J = 8.8, 5.2, 3.9 Hz, 1H), 2.53 (s, 1H), 1.85–1.71 (m, 1H), 1.45 (s, 4H), 1.37 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 156.4, 140.5, 133.5, 128.9, 128.4, 128.3, 128.3, 127.3, 126.9, 122.8, 120.1, 117.0, 107.4, 76.7, 75.2, 70.8, 47.9, 34.8, 30.6, 28.1, 26.1. FTIR (ReactIR): 2976, 1581, 1492, 1380, 1246, 1048. HRMS (ESI) m/z: [M + Na]+ calc. for C23H26O4Na 389.1723; found 389.1740. (c = 1.75, CHCl3).
Compound 85.
A flame-dried 25 mL flask was charged with free phenol 82 (100 mg, 0.27 mmol), PhNTf2 (147 mg, 0.41 mmol, 1.5 equiv), and DMAP (4 mg, 0.03 mmol, 10 mol %). CH2Cl2 (2.7 mL, 0.1 M) was added and cooled to 0 °C. At this point, NEt3 (75 μL, 0.54 mmol, 2 equiv) was added. The ice bath was removed, and the reaction was stirred at 23 °C for 3 h. Water was added, followed by CH2Cl2. The mixture was extracted with CH2Cl2 (3×, total V = 25 mL), dried over Na2SO4, filtered, and concentrated to a residue. The residue was purified by column chromatography (30% EtOAc/hexanes) to yield triflate SI-1 as a white foam (132 mg, 98%) that was directly used in the Heck reaction.18 Triflate SI-1: 1H NMR: (400 MHz, C6D6): δ 7.65 (dd, J = 7.8, 1.8 Hz, 1H), 7.14 (s, 1H), 7.08–6.96 (m, 5H), 6.93 (td, J = 7.6, 1.3 Hz, 1H), 6.91–6.78 (m, 3H), 5.73 (d, J = 10.4 Hz, 1H), 5.50 (dt, J = 10.2, 4.1 Hz, 1H), 4.29 (s, 1H), 4.01 (d, J = 5.7 Hz, 1H), 3.51 (d, J = 14.1 Hz, 1H), 3.37 (d, J = 13.9 Hz, 1H), 3.32 (dd, J = 8.2, 5.4 Hz, 1H), 1.84 (dddd, J = 18.1, 5.1, 3.7, 2.5 Hz, 1H), 1.71 (dddd, J = 18.1, 8.2, 4.5, 1.9 Hz, 1H), 1.35 (s, 4H), 1.28 (s, 3H). 19F NMR (400 MHz, C6D6): δ–73.7. TLC (5:1 hexanes:EtOac): Rf 0.28 (UV, KMnO4).
A portion of triflate SI-1 (32 mg, 0.065 mmol), Pd(PPh3)4 (7.5 mg, 0.0065 mmol, 10 mol %), and LiCl (8.5 mg, 0.2 mmol, 3 equiv) was added to a flame-dried vial and degassed with argon. DMF (1.3 mL) and NEt3 (28 μL, 0.2 mmol, 3 equiv) were added, and the mixture was subjected to three freeze–pump–thaw cycles. The vial was then submerged in a 100 °C oil bath for 36 h. The reaction was cooled and quenched with brine. The mixture was extracted with Et2O (×3, total V = 20 mL), dried over Na2SO4, filtered, and concentrated. 1H NMR analysis of the crude mixture showed one diastereomer. The residue was purified by flash column chromatography (15% EtOAc/hexanes) to give the core system 85 as a colorless oil (24 mg, 95%).18 TLC (5:1 hexanes:EtOAc): Rf 0.28 (UV, KMnO4). 1H NMR (400 MHz, CDCl3): δ 7.33 (dd, J = 8.0, 1.7 Hz, 2H), 7.29–7.18 (m, 4H), 7.10–6.93 (m, 4H), 6.05 (ddd, J = 10.2, 4.0, 1.4 Hz, 1H), 5.73 (dt, J = 10.2, 2.5 Hz, 1H), 4.85 (s, 1H), 4.58 (ddt, J = 4.7, 3.2, 1.7 Hz, 1H), 4.53 (d, J = 5.7 Hz, 1H), 3.76 (s, 1H), 3.25 (d, J = 16.2 Hz, 1H), 3.04 (d, J = 16.2 Hz, 1H), 2.46 (d, J = 2.0 Hz, 1H), 1.24 (s, 3H), 1.10 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 144.5, 142.1, 141.0, 129.3, 128.0, 127.7, 126.4, 126.0, 124.4, 123.8, 122.4, 108.2, 80.8, 71.4, 53.0, 47.0, 37.7, 27.2, 26.3. FTIR (ReactIR): 2976, 2365, 1558, 1540, 727. HRMS (ESI) m/z: [M + Na]+ calc. for C23H24O3Na 371.1618; found 371.1610. (c = 1.0, CHCl3).
Compound 87.
Prepared as previously reported with 4-methyl salicylic acid 86 (7.61 g, 50 mmol), acetone (150 mL, 0.33 M), K2CO3 (27.6 g, 200 mmol, 4 equiv), and MeI (31 mL, 500 mmol, 10 equiv) and then 1 M aqueous NaOH (150 mL, 0.33 M) to give methyl ether 87 (8.30 g, 99%).61
Compound 88.
Prepared as previously reported with carboxylic acid 87 (8.31 g, 8.56 mmol) and thionyl chloride (83 mL, 0.6 M) and then CH2Cl2 (166 mL, 0.3 M) and Et2NH (20.6 mL, 34.2 mmol, 4 equiv) to give amide 88 (6.84 g, 62%).45
Compound 89.
Prepared as previously reported with THF (74.0 mL, 0.27 M), sec-butyl lithium (1.4 M in cyclohexane, 18.6 mL, 26.0 mmol, 1.3 equiv), tetramethylenediamine (3.87 mL, 26 mmol, 1.3 equiv), amide 88 (4.42 g) in THF (15.3 mL, 1.3 M), and DMF (1.87 mL, 24 mmol, 1.2 equiv) to give the formylated amide 89 (3.18 g, 64%).45
Compound 90.
Prepared as previously reported with formylated amide 89 (2.08 g, 8.36 mmol), 1 N HCl (38.9 mL), and glacial acetic acid (19.4 mL) to give lactol 90 (1.38 g, 85%).45
Compound 20.
Prepared as previously reported with lactol 90 (1.20 g, 6.2 mmol), acetonitrile (31 mL, 0.2 M), DBU (3.7 mL, 24.8 mmol, 4 equiv), and methyl iodide (1.5 mL, 24.8 mmol, 4 equiv) to give methyl ester 20 as a white solid (1.17 g, 90%).18,45
Thermal, Brønsted Acid, or Lewis Acid-Promoted Allylation Attempts.
Allyl pinacolboronic ester 78 (0.50 mg, 0.1 mmol) was dissolved in toluene (0.5–0.1 M) in a microwave vial charged with excess aldehyde 20. The mixture was either brought to high temperatures (>220 °C) or charged with an acid (p-TsOH or BF3 OEt2, catalytic to stoichiometric amounts) at 0 °C. In all cases, protodeborylation and decomposition of boronic ester were major pathways. No allylative reactivity was observed by 1H NMR.
Example of Trifluoroborate Potassium Salt Allylation Attempt.
Allyl pinacol boronic ester 78 (0.25 mmol) was dissolved in MeOH (1.85 mL), to which KHF2 (39 mg, 0.5 mmol) in H2O (1.85 mL) was added. The mixture was stirred until completion by TLC, which showed multiple isomers. The slurry was evaporated to a crude residue and washed with Et2O/hexanes (10 mL, 10%). The solid was extracted with acetone to give the crude trifluoroborate potassium salt mixture, which was immediately utilized in the allylation reaction. After dissolving the intermediate in CH2Cl2 (2.5 mL) and cooling to −78 °C, aldehyde 20 (excess) and BF3 OEt2 (catalytic to excess amounts) were added. Monitoring by TLC and 1H NMR to 23 °C showed trace conversion and majority decomposition events.
Example of Iridium-Catalyzed Allylation.
An intermediate allyl acetate was prepared as previously reported18 with allylic alcohol 77 (230 mg, 0.59 mmol), 4-dimethylaminopyridine (7.3 mg, 0.06 mmol, 10 mol %), CH2Cl2 (1.18 mL, 0.5 M), pyridine (142 μL, 1.77 mmol, 3 equiv), and acetic anhydride (67 μL, 0.71 mmol, 1.2 equiv) to give the allylation precursor (194 mg, 76%).18 [Ir(cod)Cl]2 (1.7 mg, 0.0025 mmol, 2.5 mol %), aldehyde 20 (excess), (R)- or (S)-SEGPHOS (3.0 mg, 0.005 mmol, 5 mol %), m-NO2BzOH (1.7 mg, 0.01 mmol, 10 mol %), and Cs2CO3 (6.5 mg, 0.02 mmol, 20 mol %) were added to a microwave vial, which was crimp-sealed and filled with argon. Allyl acetate (43 mg, 0.1 mmol) in THF (500 μL, 0.2 M) and then i-PrOH (15 μL, 0.2 mmol, 2 equiv) were added. The mixture was brought to 100 °C behind a blast shield. No allylative reactivity was observed by 1H NMR and TLC.
Example of Zinc Allylation from Allyl Chloride Attempt.
The allyl chloride was prepared as previously reported18 with allylic alcohol 77 (224 mg, 0.57 mmol), PPh3 (134 mg 0.51 mmol, 0.9 equiv), CH2Cl2 (2.85 mL, 0.2 M), and N-chlorosuccinimide (88 mg, 0.63 mmol, 1.1 equiv) to give the allylation precursor (150 mg, 65%). A flame-dried vial was charged with Zn powder (86 mg, 1.32 mmol, 3.6 equiv) and LiCl (11 mg, 0.26 mmol, 0.7 equiv). After flushing with argon, THF was added, followed by one drop of dibromoethane. The previously described allyl chloride (150 mg, 0.37 mmol) was added dropwise in THF. Titration at this time only showed <10% conversion to the allyl zinc species. Zn and LiCl equivalents as well as solvent molarity were screened. Benzaldehyde or aldehyde 20 (excess) was added neat or the solution was transferred into a solution of benzaldehyde. Trace conversion with a mixture of isomers was observed by 1H NMR.
Example of Palladium-Catalyzed Borylation from Allyl Acetate Attempts.
The intermediate acetate or chloride from allyl alcohol 77 was produced as described for the iridium or zinc allylation attempts (vide infra). The allyl precursor (0.1 mmol) was treated with Pd(PPh3)4 (12 mg, 0.01 mmol, 10 mol %) (or Pd2(dba)3) in DMSO (250 μL, 0.4 M) with B2(OH)4 (11 mg, 0.12 mmol, 1.2 equiv). No conversion was observed. Degassed chloroform (1 mL) was added, and the mixture was moved to a vial protected with argon. The mixture was washed with degassed brine (3 × 1 mL). The organic layer was transferred to a vial charged with Na2SO4 under argon and dried for 30 min. The dried organic layers were moved to another oven-dried vial with a stir bar and treated with an excess of benzaldehyde or aldehyde 20 overnight. Both the chloride and acetate were examined, as well as diverse catalyst loadings and boron equivalents. No allylative reactivity was observed by 1H NMR.
Example of Borinic Ester Formation from Allyl Pinacolboronic Ester 17.
A flame-dried vial was charged with allyl pinacolboronic ester 78 (50 mg, 0.1 mmol) in THF (1 mL, 0.1 M) and was cooled to −78 °C. Alkyllithium (n-butyl or methyl, 1.1 equiv) was added dropwise. The yellow solution was stirred for 15 min at −78 °C. Trifluoroacetic anhydride (17 μL, 0.12 mmol, 1.2 equiv) was added dropwise, and the solution was stirred at −78 °C for 30 min. Excess aldehyde 20 was added to THF, and the reaction was allowed to warm to room temperature. No conversion was observed. The reaction was monitored and heated in intervals to reflux, upon which significant decomposition occurred, and no allylative reactivity was observed by 1H NMR.
Example of In Situ Esterification of Allyl Pinacol Boronic Ester 17.
Allyl pinacolboronic ester 78 (0.50 mg, 0.1 mmol) was dissolved in toluene (0.5–0.1 M) with an alcohol additive (tert-butanol or methanol, 5–20 equiv) or in the respective alcohol in a flame-dried vial charged with excess aldehyde 20. The mixture was brought to reflux. In all cases, protodeborylation and decomposition of boronic ester were major pathways. No allylative reactivity was observed by 1H NMR.
Procedure for Optimization of Allylboronic Acid 91 (Table S14).
A dry 4 mL vial with a stir bar was charged with allyl alcohol 77 (39 mg, 0.1 mmol) and solvent(s) under argon. Pd(MeCN)4(BF4)2 and B2(OH)4 (11 mg, 0.12 mmol, 1.2 equiv) were added consecutively, and the mixture was stirred under argon. After 6 h, degassed chloroform (1 mL) was added, and the mixture was moved to a vial protected with argon. The mixture was washed with degassed brine (3 × 1 mL). The organic layer was transferred to a vial charged with Na2SO4 under argon and dried for 30 min. The dried organic layers were moved to another argon-filled oven-dried vial with a stir bar and treated with an excess of benzaldehyde overnight. Rotary evaporation of the solution gave an oil that was analyzed by 1H NMR with 1,4-dimethoxybenzene as an internal standard. Unless noted otherwise, a significant starting material could be recovered from each reaction, and the product was formed as one diastereomer.18
Compound 72.
Allylic alcohol 77 (2.42 g, 6.20 mmol, 4 equiv) was transferred to a flask as a solution in CH2Cl2, which was concentrated in vacuo on a rotary evaporator and then on a Schlenk line overnight. After blanketing with argon, degassed DMSO (8.0 mL) and MeOH (1.5 mL) were added to the flask. Once the starting material had dissolved, Pd(MeCN)4(BF4)2 (275 mg, 0.62 mmol, 40 mol %) was added, followed by bisboronic acid B2(OH)4 (667 mg, 7.44 mmol, 4.8 equiv). The mixture was placed in a room-temperature water bath to moderate an initial exotherm, and the mixture was stirred under argon for 6 h. At this time, the reaction was allowed to settle for 2 h. The mixture was transferred by a syringe and needle into an argon-purged pear flask, and all further transfers were carried out by a syringe and needle into argon-filled vessels to avoid oxygen. Degassed CHCl3 (19 mL) and a brine/water (1:1) solution (19 mL) were added to the mixture under an inert atmosphere. The organic layer was washed 3 more times with the degassed water/brine solution (19 mL × 3) and then transferred into another flask under argon to which had been added Na2SO4. After 1 h, the organic layer containing allylating agent 91 was removed from the Na2SO4 and transferred to a dry microwave vial precharged with aldehyde 20 (323 mg, 1.55 mmol) The mixture was submerged into an 85 °C oil bath and stirred overnight. At this time, the microwave vial was opened to air, and methanol (2 mL) was added. The mixture was transferred to a flask and concentrated on a rotary evaporator, followed by flash column chromatography (30% EtOAc/hexanes) to furnish the allylation product 72 as a white foam (673 mg, 80%) as well as the starting material allylic alcohol 77 (680 mg, 1.74 mmol, 1.1 equiv). TLC (3:1 hexanes:EtOAc): Rf 0.11 (UV). 1H NMR (400 MHz, C6D6): δ 7.36 (dd, J = 7.6, 1.7 Hz, 1H), 6.95–6.86 (m, 1H), 6.76–6.68 (m, 2H), 6.64 (s, 1H), 6.19 (s, 1H), 6.10–6.03 (m, 1H), 5.71 (ddd, J = 10.1, 5.4, 3.2 Hz, 1H), 5.07 (s, 1H), 4.67 (d, J = 5.8 Hz, 1H), 4.39 (td, J = 6.9, 2.8 Hz, 1H), 3.53 (d, J = 14.4 Hz, 1H), 3.36 (s, 3H), 3.13 (d, J = 14.4 Hz, 1H), 2.16 (dddd, J = 18.2, 5.6, 2.9, 1.3 Hz, 1H), 2.05 (ddt, J = 18.2, 7.2, 3.0 Hz, 1H), 1.93 (s, 3H), 1.42 (s, 3H), 1.24 (s, 3H), 1.03 (s, 10H), 0.15 (s, 3H), 0.14 (s, 3H). 13C NMR (101 MHz, C6D6): δ 166.8, 158.8, 154.8, 150.9, 146.1, 132.9, 129.6, 128.8, 128.2, 127.9, 127.3, 120.9, 119.4, 116.4, 113.9, 112.0, 107.7, 80.8, 77.4, 72.4, 55.3, 46.9, 31.5, 30.3, 27.5, 26.3, 26.3 (stacked), 25.2, 22.1, 18.7, −3.7, −4.0. FTIR (ReactIR): 1760, 1606, 1491, 1241, 1045. HRMS (ESI) m/z: [M + H]+ calc. for C32H43O6Si 551.2823; found 551.2841. (c = 0.9, MeOH).18
Compound 97.
A flask was charged with TBS-protected phenol 72 (388 mg, 0.70 mmol) and THF (7.0 mL, 0.1 M) and then cooled to −78 °C. Tetrabutylammonium fluoride (1 M in THF, 770 μL, 0.77 mmol, 1.1 equiv) was added dropwise to the clear solution to give a yellow mixture. The mixture was stirred at −78 °C for 45 min and then quenched by the addition of a 1:1 solution of water and aqueous sat. NaHCO3. The mixture was extracted with EtOAc (200 mL), washed with water (×3) and brine, then dried over Na2SO4, filtered, and concentrated. The crude product was purified by flash column chromatography (40% EtOAc/hexanes) to give free phenol 97 as a white foam (307 mg, 99%). TLC (1:1 hexanes:EtOAc): Rf 0.36 (UV, KMnO4). 1H NMR (400 MHz, CDCl3): δ 7.70 (s, 1H), 7.06 (t, J = 7.7 Hz, 1H), 6.83 (s, 1H), 6.80 (d, J = 8.1 Hz, 1H), 6.72 (s, 1H), 6.52 (t, J = 7.4 Hz, 1H), 6.02 (td, J = 6.6, 3.1 Hz, 1H), 5.73 (s, 1H), 5.47 (d, J = 10.1 Hz, 1H), 5.22 (s, 1H), 4.94 (d, J = 6.6 Hz, 1H), 4.73 (t, J = 6.6 Hz, 1H), 4.04 (s, 3H), 2.98 (d, J = 15.6 Hz, 1H), 2.40 (s, 3H), 2.38–2.27 (m, 2H), 1.49 (s, 3H), 1.45 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 168.2, 158.7, 156.4, 149.7, 147.9, 132.0, 129.2, 128.6, 127.3, 122.4, 119.4, 116.6, 116.4, 112.9, 112.4, 108.3, 80.0, 78.8, 71.8, 56.1, 44.6, 31.8, 29.7, 26.3, 24.9, 22.6. FTIR (ReactIR): 1760, 1607, 1492, 1242, 1048, 921. HRMS (ESI) m/z: [M + H]+ calc. for C26H29O6 437.1959; found 437.1969. (c = 2.6, CHCl3).18
Compound 98.
A flask was charged with free phenol 97 (306 mg, 0.70 mmol) and methanol (14 mL, 0.05 M) and then cooled to 0 °C. (Diacetoxyiodo)benzene (676 mg, 2.1 mmol, 3 equiv) was added in one portion, and the mixture was vigorously stirred at 0 °C. After 1 h, the reaction was quenched by the addition of aqueous sat. NaHCO3. Methanol was removed on a rotary evaporator, and the mixture was extracted with EtOAc (100 mL). The organic layers were washed with aqueous sat. NaHCO3, aqueous sat. Na2S2O8, and brine. The organic layers were dried over Na2SO4, filtered, and concentrated. The crude product was immediately purified by flash column chromatography (1% triethylamine, 45% EtOAc/hexanes) to give a white foam as the title p-quinone monoketal 98 (219 mg, 63%).18 TLC (1:1 hexanes:EtOAc): Rf 0.18 (UV, KMnO4). 1H NMR (400 MHz, C6D6) δ 7.01 (s, 1H), 6.87 (d, J = 3.2 Hz, 1H), 6.45–6.36 (m, 2H), 6.20 (d, J = 10.3 Hz, 1H), 5.84 (ddt, J = 10.0, 2.4, 1.3 Hz, 1H), 5.80–5.70 (m, 1H), 4.96 (s, 1H), 4.78 (d, J = 6.4 Hz, 1H), 4.42 (td, J = 6.9, 2.9 Hz, 1H), 3.49 (s, 3H), 3.28 (s, 3H), 3.19 (s, 3H), 2.96 (d, J = 14.0 Hz, 1H), 2.65 (d, J = 13.8 Hz, 1H), 2.28–2.18 (m, 4H), 2.19 (s, 4H), 2.19–2.07 (m, 3H), 1.43 (s, 3H), 1.33 (s, 3H). 13C NMR (101 MHz, C6D6): δ 185.0, 159.0, 150.4, 146.5, 142.4, 141.7, 137.5, 130.6, 129.9, 128.2, 128.0, 116.6, 114.0, 112.4, 108.1, 93.8, 80.8, 77.1, 72.1, 55.4, 50.5, 50.1, 46.2, 30.4, 29.4, 27.2, 25.1, 22.3. FTIR (ReactIR): 2990, 2364, 1700, 1652, 705. HRMS (ESI) m/z: [M + H]+ calc. for C28H33O8 497.2170; found 497.2165. (c = 1.3, MeOH).
General Procedure for Hauser Optimization (Table 2).
A flame-dried 4 mL vial was charged with t-BuOLi (1 M in THF, equiv) and cooled to −78 °C. Sulfone 27 (1 equiv) was added as a solid in one portion to give an orange-yellow mixture, which was stirred at −78 °C for 15 min. At this time, p-quinone monoketal 98 (1 equiv) was added in THF (0.33 M) dropwise to the mixture. The resulting mixture was stirred at −78 °C and allowed to slowly warm to room temperature. After 15 h, the purple mixture was cooled to 0 °C and quenched with the addition of water. The mixture was transferred into a separatory funnel with EtOAc (40 mL), and the layers were separated. The aqueous layer was acidified with 1 N HCl to a pH of 4–5 and then extracted with EtOAc (100 mL total). The red organic layer was dried over Na2SO4, filtered, and concentrated to give a red residue. The residue was purified by flash column chromatography (75% EtOAc/hexanes) to give anthraquinones 18 and 100 as two separable diastereomers.18 Reactions were run on a 0.1 mmol scale. See characterization data of the anthraquinones from the optimized reaction below.
Compounds 18 and 100.
A flame-dried 25 mL flask was charged with t-BuOLi (1 M in THF, 1.60 mL, 2.65 equiv) and cooled to −78 °C. Sulfone 27 (181 mg, 0.60 mmol, 1 equiv) was added as a solid in one portion (note: if not added as a solid as this stage, reaction yield and d.r. would significantly decrease) to give an orange-yellow mixture, which was stirred at −78 °C for 15 min. At this time, p-quinone monoketal 98 (297 mg, 0.60 mmol) was added in THF (1.80 mL, 0.33 M) dropwise to the mixture. The resulting mixture was stirred at −78 °C and allowed to slowly warm to room temperature. The color changed from orange yellow to a deep purple, indicating formation of the deprotonated anthraquinone. After 15 h, the purple mixture was cooled to 0 °C and quenched with the addition of water. The mixture was transferred into a separatory funnel with EtOAc (40 mL), and the layers were separated. The aqueous layer was acidified with 1 N HCl to a pH of 4−5 and then extracted with EtOAc (100 mL total). The red organic layer was dried over Na2SO4, filtered, and concentrated to give a red residue. The residue was purified by flash column chromatography (75% EtOAc/hexanes) to give the desired anthraquinone 18 (250 mg, 66%) as a red foam and the undesired epimer 100 (21 mg, 6%; total 72% yield of anthraquinones) as a red solid, whose crystal structure was confirmed by X-ray crystallography.18 If anthraquinone 100 was desired, the reaction could be heated to reflux for 3 h or compound 18 could be heated with > 1 equivalent t-BuOLi. Desired anthraquinone 18: TLC (EtOAc): Rf 0.35 (UV, visible red spot). 1H NMR (400 MHz, CDCl3): δ 12.90 (s, 1H), 7.85 (d, J = 1.0 Hz, 1H), 7.64 (t, J = 8.1 Hz, 1H), 7.36−7.28 (m, 2H), 6.94 (s, 1H), 6.58 (s, 1H), 6.10−5.99 (m, 2H), 5.23 (s, 1H), 4.73 (d, J = 6.6 Hz, 1H), 4.63 (td, J = 6.9, 2.7 Hz, 1H), 4.00 (s, 3H), 3.93 (s, 3H), 3.90 (s, 3H), 3.08 (d, J = 14.2 Hz, 1H), 3.01 (d, J = 14.2 Hz, 1H), 2.55−2.40 (m, 1H), 2.35 (s, 2H), 2.32 (dd, J = 4.8, 2.7 Hz, 1H), 1.40 (s, 3H), 1.34 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 189.1, 182.0, 168.3, 159.8, 158.2, 155.7, 152.3, 150.0, 147.3, 134.8, 134.7, 133.9, 129.4, 127.9, 127.8, 124.0, 120.5, 118.7, 118.6, 116.8, 114.7, 112.5, 111.8, 108.0, 81.0, 77.8, 72.1, 57.2, 56.7, 55.9, 46.5, 30.9, 29.8, 27.1, 25.2, 22.6. FTIR (ReactIR): 1761, 1670, 1607, 1237, 1048. HRMS (ESI) m/z: [M + H]+ calc. for C36H35O10 627.2225, found 627.2254. (c = 0.8, MeOH). Undesired epimer anthraquinone 100: TLC (EtOAc): Rf 0.50 (UV, visible red spot). 1H NMR (400 MHz, CDCl3): δ 13.34 (s, 1H), 7.91−7.85 (m, 2H), 7.66 (t, J = 8.1 Hz, 1H), 7.33 (dd, J = 8.5, 1.1 Hz, 1H), 7.24 (s, 1H), 6.73 (s, 1H), 5.74 (dt, J = 10.1, 4.1 Hz, 1H), 5.35 (s, 1H), 5.19 (dd, J = 10.1, 1.6 Hz, 1H), 4.18 (ddd, J = 7.7, 6.0, 3.8 Hz, 1H), 4.08 (dd, J = 6.0, 1.3 Hz, 1H), 4.01 (s, 3H), 3.97 (s, 3H), 3.96 (s, 3H), 3.58 (d, J = 13.8 Hz, 1H), 3.20 (d, J = 13.9 Hz, 1H), 2.46 (s, 4H), 2.12 (dtd, J = 18.5, 4.3, 2.0 Hz, 1H), 1.46 (s, 3H), 1.34 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 189.5, 182.1, 168.2, 159.8, 158.3, 156.3, 152.7, 150.5, 147.5, 134.6, 134.1, 133.8, 128.7, 128.2, 126.7, 123.9, 121.1, 118.9, 118.7, 116.8, 115.3, 112.0, 112.0, 107.4, 82.2, 75.4, 72.5, 57.5, 56.7, 56.0, 46.9, 33.1, 30.2, 27.8, 26.1, 22.9. FTIR (ReactIR): 1761, 1670, 1607, 1236, 1046. HRMS (ESI) m/z: [M + H]+ calc. for C36H35O10 627.2225; found 627.2252. (c = 0.7, CHCl3).
Compound 102.
Prepared as previously reported with 2,5-methoxybenzoic acid 101 (9.1 g, 50 mmol) and thionyl chloride (8 mL, 110 mmol, 2.2 equiv) and then CH2Cl2 (38 mL, 1.3 M) and Et2NH (10.34 mL, 100 mmol, 2 equiv) to give compound 102 (3.87 g, 33%).62
Compound 103.
Prepared as previously reported with THF (14.8 mL, 0.27 M), sec-butyl lithium (1.4 M in cyclohexane, 3.71 mL, 5.2 mmol, 1.3 equiv), tetramethylenediamine (774 μL, 5.2 mmol, 1.3 equiv), amide 102 (949 mg, 4 mmol) in THF (3 mL, 1.3 M), and DMF (373 μL, 4.8 mmol, 1.2 equiv) to give the formylated amide 103 (782 mg, 74%).63
Compound 104.
Prepared as previously reported with formylated amide 103 (767 mg, 2.90 mmol), 1 N HCl (13.4 mL), and glacial acetic acid (6.7 mL) to give the title lactol 104 (342 mg, 65%).64
Compound 105.
Prepared as previously reported, lactol 104 (180 mg, 1 mmol), p-toluenesulfonic acid hydrate (9.5 mg, 0.05 mmol, 5 mol %), MgSO4 (2 g, 1 g per mmol SM), and toluene (5 mL, 0.2 M) were added to a flask and reflux condenser. Thiophenol (84 μL, 0.9 mmol, 0.9 equiv) was added, and the mixture was brought to boil for 3 h. The mixture was cooled and quenched by the slow addition of aqueous saturated NaHCO3. The mixture was extracted with EtOAc (100 mL). The organic layers were washed with aqueous saturated NaHCO3, water, and brine, followed by drying over Na2SO4, filtration, and concentration on the rotary evaporator. The orange solid 105 was used directly in the next step (252 mg, 83%).65 1H NMR (400 MHz, CDCl3): δ 7.48 (dd, J = 7.9, 1.8 Hz, 2H), 7.28−7.22 (m, 3H), 7.08 (d, J = 8.8 Hz, 1H), 6.86 (d, J = 8.9 Hz, 1H), 6.59 (s, 1H), 3.95 (s, 3H), 3.87 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 167.1, 152.0, 147.8, 135.2, 134.3, 130.4, 129.0, 129.0, 118.1, 114.9, 113.3, 83.9, 56.5, 56.4. FTIR (ReactIR): 1772, 1507, 1071, 1032, 967. LRMS (ESI): calculated [M + H]+ 303.06, found 303.0.
New: Compound 106.
Sulfide 105 (230 mg, 0.76 mmol) was dissolved in CH2Cl2 (7.6 mL, 0.1 M) and cooled to 0 °C. One portion of m-chloroperoxybenzoic acid (<77%, 341 mg, 1.52 mmol, 2 equiv) was added at 0 °C. The mixture was stirred for 20 min at °C and then a second portion of m-CPBA (170 mg, 0.76 mmol, 1 equiv) was added. The mixture was stirred for 30 min afterward and then allowed to come to room temperature and stirred for 1 h. The reaction was quenched with aqueous saturated NaHCO3, and the layers were separated. The organic layer was dried over Na2SO4, filtered, and concentrated. Further purification by flash column chromatography (50% EtOAc/CH2Cl2) gave the title sulfonated phthalide 106 as a white solid (188 mg, 0.56 mmol, 74%). 1H NMR (400 MHz, CDCl3): δ 7.93 (dd, J = 8.4, 1.3 Hz, 2H), 7.70−7.66 (m, 1H), 7.56 (dd, J = 8.4, 7.4 Hz, 2H), 7.22 (d, J = 8.9 Hz, 1H), 7.02 (d, J = 8.9 Hz, 1H), 6.23 (d, J = 0.5 Hz, 1H), 3.95 (s, 3H), 3.93 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 165.6, 152.6, 149.6, 135.9, 134.8, 130.0, 129.3, 128.5, 119.6, 115.1, 114.9, 89.6, 56.9, 56.6. HRMS (ESI): calculated [M + H]+ 335.0584, found 335.0576. FTIR (ReactIR): 1796, 1510, 1335, 1107, 1033.
New: Compounds 107 and 108.
A flame-dried vial was charged with t-BuOLi (1 M in THF, 1.06 mL, 2.65 equiv) and cooled to −78 °C. Sulfone 106 (134 mg, 0.40 mmol, 1 equiv) was added as a solid in one portion to give an orange-yellow mixture, which was stirred at −78 °C for 15 min. At this time, p-quinone monoketal 98 (198 mg, 0.40 mmol) was added in THF (1.21 mL, 0.33 M) dropwise to the mixture. The resulting mixture was stirred at −78 °C and allowed to slowly warm to room temperature. The color changed from orange yellow to a deep purple, indicating formation of the deprotonated anthraquinone. After 15 h, the purple mixture was cooled to 0 °C and quenched with the addition of water. The mixture was transferred into a separatory funnel with EtOAc (40 mL), and the layers were separated. The aqueous layer was acidified with 1 N HCl to a pH of 4−5 and then extracted with EtOAc (100 mL total). The red organic layer was dried over Na2SO4, filtered, and concentrated to give a red residue. The residue was purified by flash column chromatography (90% EtOAc/hexanes) to give the desired anthraquinone 107 (108 mg, 42%) as a red solid and the undesired epimer 108 (36 mg, 14%; total 56% yield of anthraquinones) as a red solid. Compound 107: 1H NMR: (400 MHz, CDCl3): δ 12.76 (s, 1H), 7.33−7.18 (m, 5H), 7.01 (s, 1H), 6.60 (s, 1H), 6.04−5.92 (m, 2H), 5.23 (s, 1H), 4.64 (d, J = 6.7 Hz, 1H), 4.58 (td, J = 6.9, 3.0 Hz, 1H), 3.97 (s, 3H), 3.94 (s, 3H), 3.91 (s, 3H), 3.90 (s, 3H), 3.10 (d, J = 14.1 Hz, 1H), 2.96 (d, J = 14.1 Hz, 1H), 2.49 (ddt, J = 17.7, 7.3, 2.4 Hz, 1H), 2.37 (s, 3H), 2.31 (dt, J = 17.9, 3.8 Hz, 1H), 1.38 (s, 3H), 1.31 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 189.1, 182.8, 168.4, 158.2, 154.9, 154.2, 153.2, 151.2, 150.1, 147.3, 134.6, 129.7, 127.4, 126.6, 126.0, 121.8, 121.5, 120.7, 118.5, 117.1, 115.9, 112.5, 111.8, 108.0, 81.3, 77.8, 72.1, 57.4, 57.1, 57.0, 55.9, 46.5, 31.1, 29.7, 27.1, 25.2, 22.7. FTIR (ReactIR): 1756, 1732, 1700, 1652, 1560. HRMS (ESI): calculated [M + H]+ 657.2330, found 657.2318. , (c = 0.85, CHCl3). Compound 108: 1H NMR: (400 MHz, CDCl3): δ 13.23 (s, 1H), 7.84 (s, 1H), 7.34 (d, J = 9.1 Hz, 1H), 7.24 (s, 1H), 6.72 (s, 1H), 5.69 (dt, J = 9.3, 4.2 Hz, 1H), 5.35 (s, 1H), 5.12 (d, J = 10.0 Hz, 1H), 4.39−4.24 (m, 1H), 4.15 (d, J = 1.3 Hz, 1H), 3.99 (s, 3H), 3.96 (d, J = 1.5 Hz, 6H), 3.58 (d, J = 13.8 Hz, 1H), 3.17 (d, J = 13.9 Hz, 1H), 2.47 (s, 3H), 2.10 (d, J = 18.5 Hz, 1H), 1.45 (s, 3H), 1.35 (s, 3H). 13C NMR (101 MHz, CDCl3): FTIR (ReactIR): 1767, 1737, 1701, 1631, 1521. HRMS (ESI): calculated [M + H]+ 657.2330, found 657.2326. , (c = 0.075, CHCl3).
General Procedure for Optimization of Triflation (Compound 109, Table 3).
A dry vial was charged with a base and a solvent. Anthraquinone 18 (0.1 mmol to 0.01 mmol) was added in solvent dropwise. Purple color denoted deprotonation of the red anthraquinone to its corresponding phenol. The triflating reagent (3 equiv) was added afterward. Reactions were monitored by 1H NMR, as the starting material and triflate 109 cospot as a yellow mixture on TLC (EtOAc). Triflate 109, as previously reported,18 could not be completely purified by successive column chromatography (75% EtOAc in hexanes) or PTLC due to decomposition on silica and was used directly in the next step. 1H NMR: (400 MHz, CDCl3): δ 7.76−7.69 (m, 2H), 7.66 (t, J = 8.0 Hz, 1H), 7.29 (dd, J = 8.5, 1.1 Hz, 1H), 6.74 (s, 1H), 6.73 (s, 1H), 6.03 (ddd, J = 9.9, 5.5, 3.0 Hz, 1H), 5.34 (d, J = 10.7 Hz, 1H), 5.05 (s, 1H), 4.00 (s, 3H), 4.00 (s, 3H), 3.98 (s, 3H), 3.73 (d, J = 5.9 Hz, 1H), 3.58 (q, J = 6.6 Hz, 1H), 3.50−3.40 (m, 2H), 2.44 (dd, J = 12.2, 5.6 Hz, 1H), 2.39 (s, 3H), 2.24−2.16 (m, 1H), 1.44 (s, 3H), 1.43 (s, 2H).19F NMR (400 MHz, CDCl3): δ−73.8 TLC (EtOAc): Rf 0.44 (UV, visible yellow spot).
Reported: Compound 110.
A flame-dried flask was charged with sodium hydride (60% in mineral oil) (184 mg, 4.6 mmol, 20 equiv) and purged with argon. The sodium hydride was washed with hexanes (3×, 10 mL) and dried under vacuum to remove the residual solvent for 15 min. After blanketing with argon, CH2Cl2 (5.75 mL) was added, and the mixture was cooled to 0 °C. Anthraquinone 18 (143 mg, 0.23 mmol) was added in CH2Cl2 (5.75 mL) dropwise to the sodium hydride mixture to give a purple mixture. The reaction was stirred at 0 °C for 4 h. At this time, trifluoromethanesulfonic anhydride (from either an ampule or a freshly distilled from PO5) (116 μL, 0.69 mmol, 3 equiv) was added dropwise to the reaction, followed by pyridine (55 μL, 0.69 mmol, 3 equiv) to give an orange mixture. The mixture was stirred at 0 °C for 1 h and quenched by the addition of aqueous sat. NaHCO3 solution. The mixture was extracted with CH2Cl2 (50 mL), and the organic layers were washed with sat. NaHCO3 solution and brine. The organic layers were dried over Na2SO4, filtered, and evaporated to yield a mixture of the title triflate and starting unprotected anthraquinone. The triflate was purified by flash column chromatography (75% EtOAc/hexanes). The yellow/orange band was collected to give a mixture (3:1) of free anthraquinone and triflate 109. A flame-dried 100 mL flask was charged with Pd(PPh3)4 (133 mg, 0.115 mmol, 50 mol %) and oven-dried LiCl (146 mg, 3.45 mmol, 15 equiv) and then purged with argon. DMF (26 mL) was added to the solids, then the mixture of triflate and anthraquinone in DMF (20 mL), and then triethylamine (478 μL, 3.45 mmol, 15 equiv). The mixture was subjected to the freeze−pump−thaw procedure (3×). After warming to room temperature, the flask was removed from the Schlenk line and sealed tightly with parafilm and black electrical tape. The mixture was submerged into a 100 °C oil bath and stirred for 15 h. At this time, the flask was cooled to room temperature and treated with brine. The mixture was extracted with EtOAc (total 200 mL), and the organic layers were washed with water (3×) and brine (3×). The organic layers were dried over Na2SO4, filtered, and concentrated to a residue. The residue was purified on a Teledyne-ISCO CombiFlash Rf-200 UV–Vis Automated Flash Chromatography System with a RediSep Rf High Performance Gold 15.5 g HP C18 column on a 5–100% MeCN in H2O gradient to give 110 as a yellow solid (83 mg, 61% over two steps). Analysis of the crude mixture showed one diastereomer.18 TLC (EtOAc): Rf 0.41 (UV, visible yellow spot). 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 7.7 Hz, 1H), 7.63 (t, J = 8.0 Hz, 1H), 7.27 (d, 1H, 7.7 Hz), 7.21 (s, 1H), 6.91 (s, 1H), 6.68 (s, 1H), 5.91–5.79 (m, 2H), 5.06 (s, 1H), 4.80 (d, J = 2.0 Hz, 1H), 4.63 (d, J = 7.2 Hz, 1H), 4.29 (d, J = 7.2 Hz, 1H), 4.00 (s, 3H), 3.99 (s, 3H), 3.94 (s, 3H), 3.48 (d, J = 17.4 Hz, 1H), 3.18 (d, J = 17.4 Hz, 1H), 2.40 (s, 3H), 1.39 (s, 3H), 1.21 (s, 3H). 1H NMR (400 MHz, C6D6): δ 7.92 (dd, J = 7.7, 1.0 Hz, 1H), 7.04 (t, J = 8.0 Hz, 1H), 6.76 (s, 1H), 6.55 (d, J = 8.2 Hz, 1H), 6.53 (s, 1H), 6.13 (s, 1H), 5.96 (dt, J = 10.2, 1.9 Hz, 1H), 5.74 (ddd, J = 10.1, 3.9, 2.4 Hz, 1H), 5.19 (q, J = 2.3 Hz, 1H), 4.82 (s, 1H), 4.32 (d, J = 7.2 Hz, 1H), 4.22 (ddt, J = 7.4, 3.8, 1.8 Hz, 1H), 3.51 (d, J = 17.2 Hz, 1H), 3.38 (s, 3H), 3.32 (s, 3H), 3.26 (s, 3H), 3.06 (d, J = 17.2 Hz, 1H), 2.02 (s, 3H), 1.40 (s, 3H), 1.04 (s, 3H). 13C NMR (101 MHz, C6D6): δ 185.3, 181.8, 166.9, 159.8, 159.2, 158.6, 151.6, 148.2, 145.4, 137.1, 135.9, 133.3, 130.6, 130.3, 125.5, 125.2, 121.6, 119.0, 117.9, 116.9, 115.2, 113.4, 111.9, 108.4, 81.5, 73.3, 70.9, 56.3, 55.9, 55.1, 53.8, 48.8, 36.1, 26.3, 24.7, 22.1. FTIR (ReactIR): 1696, 1541, 740, 709. HRMS (ESI) m/z: [M + Na]+ calculated C36H32O9Na 631.1939, found 631.1945. (c = 0.4, CHCl3).
General Procedure for Optimization of Demethylation of 110 (Table 4).
Compound 110 (1–5 mg) was dissolved in a solvent (0.01–0.001 M) and cooled to the desired temperature. Demethylating reagent and additive were added, and the reaction was brought to the next desired temperature and monitored by TLC. The reaction was quenched upon formation of new spots and extracted with EtOAc, dried over Na2SO4, concentrated in vacuo, and analyzed by 1H NMR. Initial experiments with MgI2 OEt2 were allowed to cool for 30 min to 1 h before quenching. This gave epimerization of the C18 center as well as iodinated byproducts. Entries 13 and 14 were quenched within 15 min of removal from the heating oil bath. Compound 112 1H NMR (400 MHz, CDCl3): δ 12.64 (s, 1H), 12.12 (s, 1H), 7.78 (d, J = 7.6 Hz, 1H), 7.68 (t, J = 8.0 Hz, 1H), 7.29 (d, J = 8.4 Hz, 1H), 7.22 (s, 1H), 6.86 (s, 1H), 6.73 (s, 1H), 5.89 (ddd, J = 10.1, 3.9, 2.5 Hz, 1H), 5.74 (dt, J = 10.1, 1.8 Hz, 1H), 5.21 (s, 1H), 4.72 (d, J = 2.5 Hz, 1H), 4.70−4.63 (m, 1H), 4.34 (d, J = 7.1 Hz, 1H), 3.53 (d, J = 17.7 Hz, 1H), 3.20 (d, J = 17.7 Hz, 1H), 2.41 (s, 3H), 1.44 (s, 3H), 1.28 (s, 3H). TLC (1:1 hexanes:EtOAc): Rf 0.66 (UV, visible yellow spot).
(+)-Rubellin C (3).
A dry flask was charged with powdered magnesium (52 mg, 2.2 mmol) and iodine (278 mg, 1.1 mmol). Dry ether (10 mL, 0.1 M) was added, and the flask was covered in aluminum foil and stirred in the dark. Approximately 3 h later, the red/brown color disappeared, and the mixture was colorless. At this time, a flame-dried 20 mL microwave tube was charged with Heck product 110 (34 mg, 0.056 mmol) in toluene (9.3 mL, 0.006 M). The freshly prepared magnesium iodide etherate (7.8 mL, 0.78 mmol, 14 equiv) was added dropwise to the starting material in toluene. The purple mixture was submerged in a 100 °C oil bath (note: a microwave tube, not a flask, is required for this reaction due to the necessary temperature with a low boiling solvent) and stirred for 4 h. At this time, the flask was cooled for 10–15 min and then quenched by the addition of water. The mixture was extracted with EtOAc, and the aqueous layer was acidified to pH = 4. The aqueous layer was extracted again with EtOAc, and the organic layers were washed with aqueous sat. Na2S2O8 solution, water, and brine. The organic layers were dried over Na2SO4, filtered, and concentrated in vacuo to give the crude demethylated product 112. The product could either be quickly purified on a Teledyne-ISCO CombiFlash Rf-200 UV–Vis Automated Flash Chromatography System with a RediSep Rf High Performance Gold 15.5 g HP C18 column on a 5–100% MeCN in H2O gradient or immediately used in the next reaction. The crude mixture of 112 was dissolved in THF (2 mL), to which was added 3 N HCl (285 μL) and stirred at 40 °C in an oil bath for 2 h. After cooling, the reaction was again extracted with EtOAc and washed with water and brine, dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was purified on a Teledyne-ISCO CombiFlash Rf-200 UV–Vis Automated Flash Chromatography System with a RediSep Rf High Performance Gold 15.5 g HP C18 column on a 5–100% MeCN in H2O gradient to give the natural product (+)-rubellin C (3)18 as a yellow solid (21 mg, 67% over two steps), which matched the characterization data from the literature.2,4 TLC (EtOAc): Rf 0.63 (UV, visible yellow spot). Reverse-phase TLC (3:1 MeOH:H2O, Merck TLC silica gel 60 RP-18 F254S 5 × 10 glass plate): Rf 0.27 (UV, visible yellow spot). 1H NMR (400 MHz, CDCl3): δ 12.54 (s, 1H, O-H), 12.12 (s, 1 H, O-H), 7.79 (dd, J = 7.6, 1.2 Hz, 1H), 7.69 (t, J = 7.9 Hz, 1H), 7.55 (s, 1H, O-H), 7.31 (dd, J = 8.4, 1.1 Hz, 2H), 7.03 (s, 1H), 6.70 (s, 1H), 6.67 (s, 1H), 5.92 (dd, J = 10.1, 2.8 Hz, 1H), 5.85 (ddd, J = 10.4, 5.0, 2.0 Hz, 1H), 5.64 (s, 1H), 4.66 (s, 1H), 4.46–4.42 (m, 1H), 4.32 (t, J = 5.5 Hz, 1H), 4.13 (d, J = 19.7 Hz, 1H), 3.01 (d, J = 5.9 Hz, 1H), 2.94 (d, J = 18.5 Hz, 1H), 2.20 (s, 3H). 1H NMR (400 MHz, DMSO-d6): δ 7.79 (dd, J = 8.4, 7.5 Hz, 1H), 7.63 (dd, J = 7.6, 1.2 Hz, 1H), 7.37 (dd, J = 8.4, 1.2 Hz, 1H), 7.29 (s, 1H), 6.97 (s, 1H), 6.71 (s, 1H), 5.69–5.62 (m, 2H), 5.52 (dd, J = 10.0, 3.0 Hz, 1H), 5.14 (d, J = 4.1 Hz, 1H), 5.05 (d, J = 6.3 Hz, 1H), 4.45 (d, J = 18.6, 1H), 4.17 (d, J = 4.6 Hz, 1H), 4.09 (t, J = 5.3 Hz, 1H), 3.90 (d, J = 3.3 Hz, 1H), 3.08 (d, J = 18.6 Hz, 1H), 2.36 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 191.4, 182.5, 166.8, 162.5, 161.2, 157.2, 156.4, 148.9, 146.6, 140.7, 137.2, 133.4, 127.0, 126.7, 125.7, 124.1, 119.7, 119.2, 116.6, 115.6, 115.5, 113.5, 110.0, 84.6, 67.5, 65.3, 52.1, 46.4, 37.5, 21.7. FTIR (ReactIR): 1740, 1665, 1626, 890, 730. HRMS (ESI) m/z: [M – H]− calc. C30H21O9 525.1191; found 525.1184. (c = 0.3, MeOH). Reported: +193.6° (c = 0.5, MeOH); +220° (c = 0.1, MeOH).2,4
Supplementary Material
ACKNOWLEDGMENTS
Financial support was provided by W. W. Caruth, Jr., Endowed Scholarship, Welch Foundation (I-1748), the National Institutes of Health (R01GM102604), the American Chemical Society Petroleum Research Fund (59177-ND1), Teva Pharmaceuticals Marc A. Goshko Memorial Grant (60011-TEV), and the Sloan Research Fellowship. J.A.G. acknowledges the NIH Pharmacological Sciences Training Grant (GM007062) for funding. The authors thank Vincent Lynch (UT Austin) for X-ray crystal structural analysis, Hamid Baniasadi (UTSW) for high-resolution mass spectrometry assistance, and Jef De Brabander (UTSW), Joseph Ready (UTSW), Chuo Chen (UTSW), Tian Qin (UTSW), and Myles Smith (UTSW) for productive discussions. The authors also thank our diverse collection of lab members for creating an environment that supported the success of this project.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.1c00920.
Spectroscopic data (PDF)
Crystallographic data (PDF)
Contributor Information
Jackson A. Gartman, Department of Biochemistry, UT Southwestern Medical Center, Dallas, Texas 75390-9038, United States.
Uttam K. Tambar, Department of Biochemistry, UT Southwestern Medical Center, Dallas, Texas 75390-9038, United States.
REFERENCES
- (1).Arnone A; Camarda L; Nasini G; Assante G Secondary Mould Metabolites. Part 15. Structure Elucidation of Rubellins A and B, two Novel Anthraquinone Metabolites from Mycosphaerella rubella. J. Chem. Soc., Perkin Trans. 1 1986, 255–260. [Google Scholar]
- (2).Arnone A; Nasini G; Camarda L; Assante G Secondary Mould Metabolites. XXV. The Structure of Rubellins C and D, Two Novel Anthraquinone Metabolites from Mycosphaerella rubella. Gazz. Chim. Ital. 1989, 119, 35–39. [Google Scholar]
- (3).Heiser I; Sachs E; Liebermann B Photodynamic oxygen activation by Rubellin D, a phytotoxin produced by Ramularia collo-cygni. Phys. Mol. Plant Path. 2003, 62, 29–36. [Google Scholar]
- (4).Miethbauer S; Haase S; Schmidtke K-U; nther W; Heiser I; Liebermann B Biosynthesis of photodynamically active rubellins and structure elucidation of the new anthraquinone derivatives produced by Ramularia collo-cygni. Phytochemistry 2006, 67, 1206–1213. [DOI] [PubMed] [Google Scholar]
- (5).Miethbauer S; nther W; Schmidtke K-U; Heiser I; Gräfe S; Gitter B; Liebermann B. Uredinorubellins and Caeruleoramularin, Photodynamically Active Anthraquinone Derivatives Produced by Two Species of the Genus Ramularia. J. Nat. Prod. 2008, 71, 1371–1375. [DOI] [PubMed] [Google Scholar]
- (6).Isaka M; Palasarn S; Tobwor P; Boonruangprapa T; Tasanathai K Bioactive Anthraquinone Dimers from the Leafhopper Pathogenic Fungus Torrubiella sp. BCC 28517 J Antibiot. 2012, 65, 571–574. [DOI] [PubMed] [Google Scholar]
- (7).Hemphill CFP; Dalteos G; Hamacher A; Kassack MU; Lin W; Mándi A; Kurtán; Proksch, P. Absolute Configuration and AntiTumor Activity of Torrubiellin B. Tetrahedron Lett. 2015, 56, 4430–4433. [Google Scholar]
- (8).Zhang C-H; Yao D-L; Li C-S; Luo J; Jin M; Zheng M-S; Lin Z-H; Jin T-F; Li G Cytotoxic Anthraquinone Dimers from Melandrium firmum. Arch. Pharm. Res. 2015, 38, 1033–1037. [DOI] [PubMed] [Google Scholar]
- (9).Miethbauer S; Heiser I; Liebermann B The Phytopathogenic Fungus Ramularia collo-cygni Produces Biologically Active Rubellins on Infected Barley Leaves. J. Phytopathol. 2003, 151, 665–668. [Google Scholar]
- (10).Heiser I; Hess M; Schmidtke K-U; Vogler U; Miethbauer S; Liebermann B Fatty Acid Peroxidation by Rubellin B, C, and D, Phytotoxins Produced by Ramularia collo-cygni. Physiol. Mol. Plant Pathol. 2004, 64, 135–143. [Google Scholar]
- (11).Morgan BJ; Mulrooney CA; Kozlowski MC Perlenequinone Natural Products: Evolution of the Total Synthesis of Cercosporin. J. Org. Chem. 2010, 75, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Miethbauer S; Gaube F; Möllmann U; Dahse H-M; Schmidtke M; Gareis M; Pickhardt M; Liebermann B Antimicrobial, Antiproliferative, Cytotoxic, and Tau Inhibitory Activity of Rubellins and Caeruleoramularin Produced by the Phytopathogenic Fungus Ramularia collo-cygni. Planta. Med. 2009, 75, 1523–1525. [DOI] [PubMed] [Google Scholar]
- (13) (a).Calcul L; Zhang B; Jinwal UK; Dickey CA; Baker BJ Natural Products as a rich source of tau-targeting drugs for Alzheimer’s disease. Future Med. Chem. 2012, 4, 1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Vaquer-Alicea J; Diamond MI Propagation of Protein Aggregation of Neurodegenerative Diseases. Annu. Rev. Biochem. 2019, 88, 785–810. [DOI] [PubMed] [Google Scholar]
- (14).Overman LE; Pennington LD A new strategy for synthesis of attached rings. Can. J. Chem. 2000, 78, 732–738. [Google Scholar]
- (15).Movassaghi M; Ahmad OK; Lathrop SP Directed Heterodimerization: Stereocontrolled Assembly via Solvent-Caged Unsymmetrical Diazene Fragmentation. J. Am. Chem. Soc. 2011, 133, 13002–13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Daub ME; Prudhomme J; Roch KL; Vanderwal CD Synthesis and Potent Antimalarial Activity of Kalihinol B. J. Am. Chem. Soc. 2015, 137, 4912–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Huffman BJ; Chen S; Schwarz JL; Plata RE; Chin EN; Lairson LL; Houk KN; Shenvi RA Electronic complementarity permits hindered butenolide heterodimerization and discovery of novel cGAS/STING pathway antagonists. Nat. Chem. 2020, 12, 310–317. [DOI] [PubMed] [Google Scholar]
- (18).Gartman JA; Tambar UK Total Synthesis of (+)-Rubellin C. Org. Lett. 2020, 22, 9145–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19) (a).Abelman MM; Oh T; Overman LE Intramolecular Alkene Arylations for Rapid Assembly of Polycyclic Systems Containing Quaternary Centers. A New Synthesis of Spirooxindoles and Other Fused and Bridged Ring Systems. J. Org. Chem. 1987, 52, 4130–4133. [Google Scholar]; (b) Zhang Y; O’Connor B; Negishi E Palladium-Catalyzed Procedures for [3+2] Annulation via Intramolecular Alkenylpalladation and Arylpalladation. J. Org. Chem. 1988, 53, 5588–5590. [Google Scholar]
- (20).Lachance H; Hall DG; Denmark SE Allylboration of Carbonyl Compounds. In Organic Reactions; Wiley, 2012; Vol. 73, pp 1–575. [Google Scholar]
- (21).Hall DG New Preparative Methods for Allylic Boronates and Their Application in Stereoselective Catalytic Allylborations. Pure Appl. Chem. 2008, 80, 913–927. [Google Scholar]
- (22).Li M; Li Y; Ludwik KA; Sandusky ZM; Lannigan DA; O’Doherty AG Stereoselective Synthesis and Evaluation of C6”-Substituted 5a-Carbasugar Analogues of SL0101 as Inhibitors of RSK1/2. Org. Lett. 2017, 19, 2410–2413. [DOI] [PubMed] [Google Scholar]
- (23).Wang ZX; Miller SM; Anderson OP; Shi Y A Class of C2 and Pseudo C2 Symmetric Ketone Catalysts for Asymmetric Epoxidation. Conformation Effect on Catalysis. J. Org. Chem. 1999, 64, 6443–6458. [Google Scholar]
- (24).Audia JE; Boisvert L; Patten AD; Villalobos A; Danishefsky SJ Synthesis of Two Useful, Enantiomerically Pure Derivatives of (S)-4-Hydroxy-2-cyclohexenone. J. Org. Chem. 1989, 54, 3738–3740. [Google Scholar]
- (25).Takahashi K; Ishiyama T; Miyaura N A borylcopper species generated from bis(pinacolato)diboron and its additions to alpha,beta-unsatured carbonyl compounds and terminal alkynes. J. Organomet. Chem. 2001, 625, 47–53. [Google Scholar]
- (26).Cano R; Ramón DJ; Yus M Impregnated Copper on Magnetite as Recyclable Catalyst for the Addition of Alkoxy Diboron Reagents to C–C Double Bonds. J. Org. Chem. 2010, 75, 3458–3460. [DOI] [PubMed] [Google Scholar]
- (27).Danishefsky SJ; Uang BJ; Quallich G Total Synthesis of Vineomycin B2 Aglycon. J. Am. Chem. Soc. 1984, 106, 2453–2455. [Google Scholar]
- (28).Kuroda S; Oda M; Syumiya HO; Shaheen SMI; Miyatake R; Nishikawa T; Yoneda A; Tanaka T; Mouri M; Kyogoku M Endoperoxidation of 9,10-bis(1-hydorxyalkyl)-anthracenes and successive formation of 9,10-anthraquinone under Grignard reaction conditions. Heterocycles 2004, 62, 153–159. [Google Scholar]
- (29).Pullella GA; Wdowiak AP; Sykes ML; Lucantoni L; Sukhoverko KV; Zulfiqar B; Sobolev AN; West NP; Mylne JS; Avery VM; Piggott MJ Total Synthesis of the Antimalarial Ascidian Natural Product Albopunctatone. Org. Lett. 2019, 21, 5519–5523. [DOI] [PubMed] [Google Scholar]
- (30).Tietze LF; Gericke KM; Schuberth I Synthesis of Highly Functionalized Anthraquinones and Evaluation of Their Antitumor Activity. Eur. J. Org. Chem. 2007, 2007, 4563–4577. [Google Scholar]
- (31).Simoneau B; Brassard P A Convenient Synthesis of Naturally Occurring Quinizarines. Tetrahedron 1988, 44, 1015–1022. [Google Scholar]
- (32).Wurm G; Göbler B Reaction of Napthol Derivatives with CuCl-O2: A Versatile Synthesis of Juglone. Arch. Pharm. 1987, 320, 564–566. [Google Scholar]
- (33).Wheeler AS; Naiman B Hydroxy-naphthoquinone studies. V. Derivatives of 2-bromo-5-hydroxy-1,4-naphthoquinone (monobromojuglone). J. Am. Chem. Soc. 1922, 44, 2331–2334. [Google Scholar]
- (34).Hauser FM; Rhee RP 4H-Anthra[1,2-b]pyran Antibiotics. Total Synthesis of the Methyl Ether of Kidamycinone. J. Am. Chem. Soc. 1979, 101, 1628–1629. [Google Scholar]
- (35).Mal D; Pahari P Recent Advances in the Hauser Annulation. Chem. Rev. 2007, 107, 1892–1918. [DOI] [PubMed] [Google Scholar]
- (36).Brimble MA; Caprio V; Johnston AD; Sidford M Synthesis of Arylspiroketals Related to the Papulacandins via Generation of Phthalide Oxycarbenium Ions. Synthesis 2001, 2001, 0855–0862. [Google Scholar]
- (37).Bates MA; Sammes PG; Thomson GA Synthesis of the C-Glycoside Fragment of Nogalamycin and some Nogalamycin Precursors. J. Chem. Soc. Perkin Trans. 1 1988, 3037–3045. [Google Scholar]
- (38).Yu M; Danishefsky SJ A Direct Route to Fluostatin C by a Fascinating Diels-Alder Reaction. J. Am. Chem. Soc. 2008, 130, 2783–2785. [DOI] [PubMed] [Google Scholar]
- (39).Russell RA; Warrener RN The Regiospecific Preparation of 1,4-Dioxygenated Anthraquinones: A New Route to Islandicin, Digitopurpone, and Madeirin. J. Chem. Soc. Chem. Commun. 1981, 108–110. [Google Scholar]
- (40).Izquierdo J; Orue A; Scheidt KA A Dual Lewis Base Activation Strategy for Enantioselective Carbene-Catalyzed Annulations. J. Am. Chem. Soc. 2013, 135, 10634–10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Piller FM; Metzger A; Schade MA; Haag BA; Gavryushin A; Knochel P Preparation of polyfunctional arylmagnesium, arylzinc, and benzylic zinc reagents by using magnesium in the presence of LiCl. Chem.–Eur. J. 2009, 15, 7192–7202. [DOI] [PubMed] [Google Scholar]
- (42) (a).Schuster DI; Rao JM Alkyl Substitutent Effects on the Photorearrangement of Cyclohex-2-en-1-ones to Lumiketones. J. Org. Chem. 1981, 46, 1515–1521. [Google Scholar]; (b) Nicolaou KC; Gray DLF; Montagnon T; Harrison ST Oxidation of Silyl Enol Ethers by Using IBX and IBX • N-oxide Complexes: a Mild and Selective Reaction for the Synthesis of Enones. Angew. Chem., Int. Ed. 2002, 41, 996–1000. [DOI] [PubMed] [Google Scholar]; (c) Lu Y; Nguyen PL; Lévaray N; Lebel H Palladium-Catalyzed Saegusa–Ito Oxidation: Synthesis of a,b-Unsaturated Carbonyl Compounds from Trimethylsilyl Enol Ethers. J. Org. Chem. 2013, 78, 776–779. [DOI] [PubMed] [Google Scholar]; (d) Kopp S; Schweizer WB; Altmann K-H Total Synthesis of Valerenic Acid. Synlett 2009, 11, 1769–1772. [Google Scholar]
- (43).Selander N; Paasch JR; Szabó KJ Palladium-Catalyzed Allylic C-OH Functionalization for Efficient Synthesis of Functionalized Allylsilanes. J. Am. Chem. Soc. 2011, 133, 409–411. [DOI] [PubMed] [Google Scholar]
- (44).Liang S; Paquette LA; Kappe O; et al. Palladium-Catalyzed Intramolecular Cyclization of Vinyl and Aryl Triflates. Associated Regioselectivity of the β-Hydride Elimination Step. Acta Chem. Scand. 1992, 46, 597–605. [DOI] [PubMed] [Google Scholar]
- (45) (a).Mamidyala SK; Ramu S; Huang JX; Robertson AAB; Cooper MA Efficient synthesis of anacardic acid analogues and their antibacterial activities. Bioorg. Med. Chem. Lett. 2013, 23, 1667–1670. [DOI] [PubMed] [Google Scholar]; (b) Mamidyala SK; Cooper MA Probing the reactivity of o-phthalaldehydic acid/methyl ester: synthesis of N-isoindolinones and 3-arylaminophthalides. Chem. Commun. 2013, 49, 8407–8409. [DOI] [PubMed] [Google Scholar]
- (46).Batey RA; Thadani AN; Smil DV Potassium Allyl- and Crotyltrifluoroborates: Stable and Efficient Agents for Allylation and Crotylation. Tetrahedron Lett. 1999, 40, 4289–4292. [Google Scholar]
- (47).Lou S; Moquist PN; Schaus SE Asymmetric Allylboration of Ketones Catalyzed by Chiral Diols. J. Am. Chem. Soc. 2006, 128, 12660–12661. [DOI] [PubMed] [Google Scholar]
- (48).Kim IS; Ngai M-Y; Krische MJ Enantioselective Iridium-Catalyzed Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level via Transfer Hydrogenative Coupling of Ally acetate: Departure from Chirally Modified Allyl Metal Reagents in Carbonyl Addition. J. Am. Chem. Soc. 2008, 130, 14891–14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Fandrick KR; Fandrick DR; Gao JJ; Reeves JT; Tan Z; Li W; Song JJ; Lu B; Yee NK; Senanayake CH Mild and General Zinc-Alkoxide-Catalyzed Allylations of Ketones with Allyl Pinacol Boronates. Org. Lett. 2010, 12, 3748–3751. [DOI] [PubMed] [Google Scholar]
- (50).Harvey NL; Krysiak J; Chamni S; Cho SW; Sieber SA; Romo D Synthesis of (±)-Spongiolactone Enabling Discovery of a More Potent Derivative. Chem.–Eur. J. 2015, 21, 1425–1428. [DOI] [PubMed] [Google Scholar]
- (51).Chen JL-Y; Scott HK; Hesse MJ; Willis CL; Aggarwal VK Highly Diastereoand Enantioselective Allylboration of Aldehydes using α-Substituted Allyl/Crotyl Pinacol Boronic Esters via in Situ Generated Borinic Esters. J. Am. Chem. Soc. 2013, 135, 5316–5319. [DOI] [PubMed] [Google Scholar]
- (52).Wang D; Szabó KJ Copper-Catalyzed, Stereoselective Cross-Coupling of Cyclic Allyl Boronic Acids with α-Diazoketones. Org. Lett. 2017, 19, 1622–1625. [DOI] [PubMed] [Google Scholar]
- (53).Raducan M; Alam R; Szabó KJ Palladium-Catalyzed Synthesis and Isolation of Functionalized Allylboronic Acids: Selective, Direct Allylboration of Ketones. Angew. Chem., Int. Ed. 2012, 51, 13050–13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Larsson JM; Szabó KJ Mechanistic Investigation of the Palladium-Catalyzed Synthesis of Allylic Silanes and Boronates from Allylic Alcohols. J. Am. Chem. Soc. 2013, 135, 443–455. [DOI] [PubMed] [Google Scholar]
- (55).Cabri W; Candiani I; DeBernardinis S; Francalanci F; Penco S; Santo R Heck Reaction of Anthraquinone Derivatives: Ligand, Solvent, and Salt Effects. J. Org. Chem. 1991, 56, 5796–5800. [Google Scholar]
- (56) (a).Badubri F; De Palma D; Farinola GM; Ragni R; Naso F Synthesis of Functionalized Anthraquinones via Coupling Reactions of 2,6-Diiodo-1,5-dioctyloxy-9,10-anthraquinone. Synthesis 2008, 8, 1227–1232. [Google Scholar]; (b) Mahal A; Villinger A; Langer P Site-Selective Arylation of Alizarin and Purpurin Based on Suzuki-Miyaura Cross-Coupling Reactions. Eur. J. Org. Chem. 2011, 2011, 2075–2087. [Google Scholar]
- (57).Jutand A; Mosleh A Rate and Mechanism of Oxidative Addition of Aryl Triflates to Zerovalent Palladium Complexes. Evidence for the Formation of Cationic (sigma-Aryl)palladium Complexes. Organometallics 1995, 14, 1810–1817. [Google Scholar]
- (58).Woo CM; Gholap SL; Lu L; Kaneko M; Li Z; Ravikumar PC; Herzon SB Development of Enantioselective Synthetic Routes to (−)-Kinamycin F and (−)-Lomaiviticin Aglycon. J. Am. Chem. Soc. 2012, 134, 17262–17273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Sloman DL; Bacon JW; Porco JA Total Synthesis and Absolute Stereochemical Assignment of Kibdelone C. J. Am. Chem. Soc. 2011, 133, 9952–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Clavaguera F; Duyckaerts C; Haïk S Prion-like properties of Tau assemblies. Curr. Opin. Neurobiol. 2020, 61, 49–57. [DOI] [PubMed] [Google Scholar]
- (61).Snider BB; Gao X Efficient Syntheses of Rugulosin Analogues. J. Org. Chem. 2005, 70, 6863–6869. [DOI] [PubMed] [Google Scholar]
- (62).da Frota LCRM; Schneider C; de Amorim MB; da Silva AJM; Snieckus V Chemoselective Ruthenium-Catalyzed C-O Bond Activation: Orthogonality of Nickeland Palladium-Catalyzed Reactions for the Synthesis of Polyaryl Fluorenones. Synlett 2017, 28, 2587–2593. [Google Scholar]
- (63).Sibi MP; Altintas N; Snieckus V Benzamide directed ortho metalation: A route to the a/b ring synthon of daunomycinone. Tetrahedron 1984, 40, 4593–4596. [Google Scholar]
- (64).Freskos JN; Morrow GW; Swenton JS Synthesis of functionalized hydroxyphthalides and their conversion to 3-cyano-1(3H)-isobenzofuranones. The Diels-Alder reaction of methyol 4,4-diethoxybutynoate and cyclohexadienes. J. Org. Chem. 1985, 50, 805–810. [Google Scholar]
- (65).Wang S; Kraus GA Annulations with Butenolides and Phthalides: New Entries to Isocoumarins, 3,4-Dihydroisocoumarins, and Benzofurans. Synthesis 2020, 52, 2821–2827. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.